
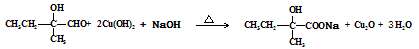
 ����2�֣�
����2�֣�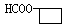 ��
�� �����������֣���2�֣�
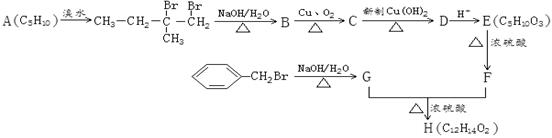
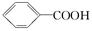
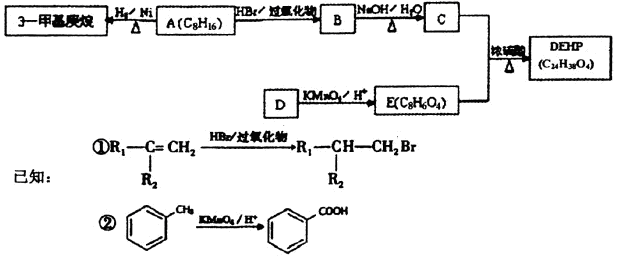
�����������֣���2�֣�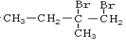 ���������Ľṹ������֪A��B�Ľṹ����B��C��D��ѧ������Ϥ�Ĵ���ȩ����������������پ��ữ�õ���E���ǻ���
���������Ľṹ������֪A��B�Ľṹ����B��C��D��ѧ������Ϥ�Ĵ���ȩ����������������پ��ữ�õ���E���ǻ���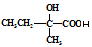 ����
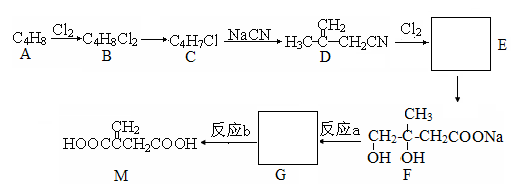
���� ֪GӦ��Ϊ���״�����G�ܺ�F��Ӧ����H֪F�����Ȼ����Ӷ���֪E��F��ת��ӦΪ��ȥ��Ӧ���ٸ�������F�ķ��ӽṹ�к���������֪F�Ľṹ��CH3CH��C(CH3)COOH�������Ƴ�H��
֪GӦ��Ϊ���״�����G�ܺ�F��Ӧ����H֪F�����Ȼ����Ӷ���֪E��F��ת��ӦΪ��ȥ��Ӧ���ٸ�������F�ķ��ӽṹ�к���������֪F�Ľṹ��CH3CH��C(CH3)COOH�������Ƴ�H��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

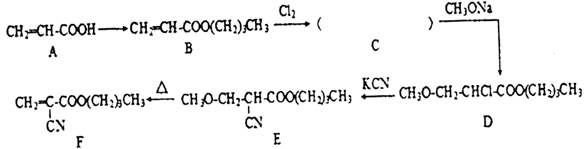
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 >H2CO3>
>H2CO3>  > HCO3�����ۺϿ��Ƿ�Ӧ���ת���ʺ�ԭ�ϳɱ������أ���
> HCO3�����ۺϿ��Ƿ�Ӧ���ת���ʺ�ԭ�ϳɱ������أ���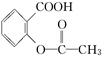 ת��Ϊ
ת��Ϊ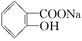 ����ѷ�����( )
����ѷ�����( )| A����ϡH2SO4���Ⱥ���������NaOH��Һ |
| B����ϡH2SO4���Ⱥ���������Na2CO3��Һ |
| C����������NaOH��Һ���Ⱥ���ͨ������CO2 |
| D����������NaOH��Һ���Ⱥ��ټ�������H2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
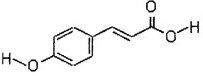
| A��������Ӧ | B����ȥ��Ӧ | C���Ӿ۷�Ӧ | D��ˮ�ⷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
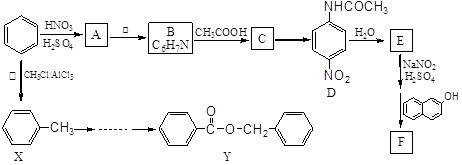
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
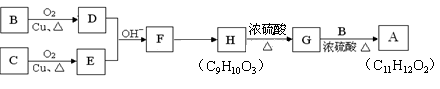
�鿴�𰸺ͽ���>>
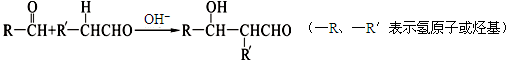
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
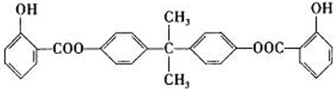 )��һ�����������ռ�����ϳ�·�����£�
)��һ�����������ռ�����ϳ�·�����£�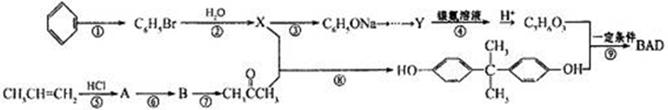
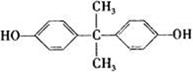 ��˵���У���ȷ����______�����ţ���
��˵���У���ȷ����______�����ţ���| A���˴Ź���������ʾ4��壬�ҷ������Ϊ1:2:2:3 |
| B������FeCl3������ɫ��Ӧ |
| C������NaHCO3��Һ������Ӧ |
| D��1mol�������������2mol Br2����ȡ����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
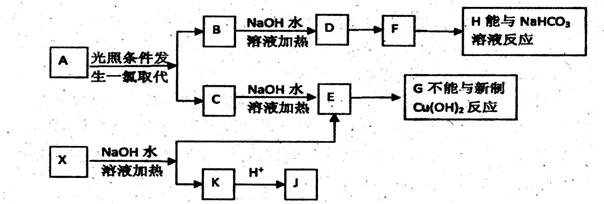
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com