
|
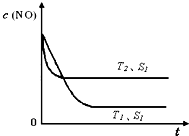
T��ʱ��1 L�ܱ�������A������B���巴Ӧ����C���壮��Ӧ������A��B��CŨ�ȱ仯��ͼ1��ʾ�������������������䣬�¶ȷֱ�ΪT1��T2ʱ��B������ٷֺ�����ʱ��Ĺ�ϵ��ͼ2��ʾ�������н�����ȷ����
| |
A�� |
�ڴ�ƽ����������������䣬����ѹǿ��ƽ��������Ӧ�����ƶ� |
B�� |
���������������䣬�����¶ȣ������淴Ӧ���ʾ�������A��ת�������� |
C�� |
�ڴ�ƽ�����ѹǿ���䣬ͨ��ϡ�����壬ƽ��������Ӧ�����ƶ� |
D�� |
T��ʱ������0.3 mol��L��1��A��0.1 mol��L��1��B��0.4 mol��L��1��C��Ӧ���ﵽƽ���C��Ũ��Ϊ0.4 mol��L��1 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?��ɽ��ģ��2012��2��27���������ƽ��롰200��������ʱ����������β���ѳ�Ϊ��Ҫ�Ŀ�����Ⱦ�
��2012?��ɽ��ģ��2012��2��27���������ƽ��롰200��������ʱ����������β���ѳ�Ϊ��Ҫ�Ŀ�����Ⱦ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2012?�˴�ģ�⣩�¶�ΪTʱ����4L�����ܱ������г���2.0molPCl5����ӦPCl5��g��?PCl3��g��+Cl2��g������һ��ʱ���ﵽƽ�⣮��Ӧ�����вⶨ�IJ������ݼ��±���
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
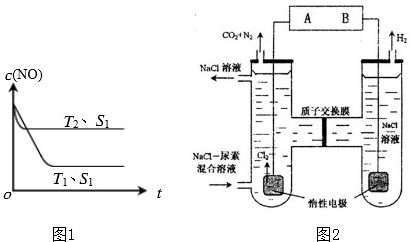 ����β���ѳ�Ϊ��Ҫ�Ŀ�����Ⱦ�
����β���ѳ�Ϊ��Ҫ�Ŀ�����Ⱦ�| ʵ���� | �¶ȡ� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ ʱ��/min | ||
| CO | H2O | H2 | CO | |||
| 1 | 650 | 4 | 2 | 1.6 | 2.4 | 6 |
| 2 | 900 | 2 | 1 | 0.4 | 1.6 | 3 |
| 3 | 900 | a | b | c | d | t |
| a |
| b |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
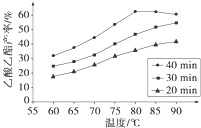 ��ҵ�ϳ����ô�����Ҵ��ϳ��л��ܼ�����������CH3COOH��l��+C2H5OH��l��
��ҵ�ϳ����ô�����Ҵ��ϳ��л��ܼ�����������CH3COOH��l��+C2H5OH��l��| ŨH2SO4 |
| �� |
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
| n��CO�� | n��H2O�� | n��H2�� | n��CO2�� | |
| A | 1 | 5 | 2 | 3 |
| B | 2 | 2 | 1 | 1 |
| C | 3 | 3 | 0 | 0 |
| D | 0.5 | 2 | 1 | 1 |
| E | 3 | 1 | 2 | 1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ĵ�ʡ��֦����2012��2013ѧ���һ��ѧ����ĩ���м�⻯ѧ���� ���ͣ�058
��t���£���1 L�����ܱ�ϵ�з�����Ӧ��CO(g)��H2O(g)![]() CO2(g)��H2(g)����H��0������Ӧ�����ʼŨ�ȷֱ�Ϊ��c(CO)��5.00 mol/L��c(H2O)��6.00 mol/L��c(CO2)��0��c(H2)��1.50 mol/L���ﵽƽ���c(CO)Ũ��Ϊ2.00 mol/L��
CO2(g)��H2(g)����H��0������Ӧ�����ʼŨ�ȷֱ�Ϊ��c(CO)��5.00 mol/L��c(H2O)��6.00 mol/L��c(CO2)��0��c(H2)��1.50 mol/L���ﵽƽ���c(CO)Ũ��Ϊ2.00 mol/L��
��
(1)����ƽ���������ƽ��Ħ��������
(2)����t���£���Ӧ�����ʼŨ�ȷֱ�Ϊ��c(CO)��3.00 mol/L��c(H2O)��3.00 mol/L����������ʼŨ�Ⱦ�Ϊ0����ﵽƽ��ʱH2Oת���ʣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com