
��ҵ��ⱥ��ʳ��ˮ������������Ư�ۣ������еķ�Ӧ��
�� 2NaCl+2H2O Cl2��H2+2NaOH
Cl2��H2+2NaOH
�� 2Ca(OH)2��ʯ���飩+ 2Cl2 =CaCl2+Ca(ClO)2+2H2O
ij���ղ�Ư�ۣ�����80%����Ҫ����Ca(OH)2)20�֣���֪���Тٲ���Ӧ��ת����90%,���Тڲ�ת����85����
��1��д���ڷ�Ӧ�����ӷ���ʽ����˫���ű������ת����Ŀ
��2����ʽ����ó������ľ��ƺ��NaCl����Ħ��������ѧ����ֵ������С�������λ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ¦���е���У�߶������л�ѧ���������棩 ���ͣ�ѡ����
���е����������ĵ����
A�����ڵ�NaOH B����� C��ʯī�� D��KNO3����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���������������ʡ��У��һ��ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ij��ɫ����ǿ������Һ�У��ܴ���������������ǣ� ��
A��Na+��K+��SO42-��.HCO3- B��Cu2+��K+��SO42- ��NO3-
C��Na+��K+��SO42-��Cl- D��NH4+��K+��Cl-��NO3-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���������������ʡ��У�߶���ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ���ǣ� ��
A������������������ڷ�Ӧ���������ʱ����H<0
B���������������������£�ʹ�ô������Ըı䷴Ӧ����
C����H<0����S>0�ķ�Ӧ�ڵ���ʱ�����Է�����
D��һ����ѧ��Ӧ�ġ�Hֻ�뷴Ӧ��ϵ��ʼ̬����̬�йأ����뷴Ӧ��;����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���������������ʡ��У�߶���ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��1.0 mol��L-l NaOH��Һ�к�ijŨ��H2 SO4��Һʱ����pH������NaOH��Һ�����(V)��ϵ��ͼ��ʾ����ԭ������Һ�����ʵ���Ũ�Ⱥ�ǡ���к�ʱ��Һ��������ֱ��ǣ������Ϻ���Һ�����Ϊ�������֮�ͣ��� ��
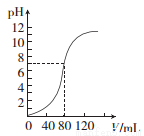
A��0.5 mol��L-1��160 mL B��1.0 mol��L-1��160 mL
C��0.5 mol��L-1��80 ml�� D��1.0 mol��L-1��80 ml��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ��һ�����л�ѧ���������棩 ���ͣ������
��1��14.2gC12��_________���������ӣ�_______Ħ����ԭ�ӣ���״������ռ�����Ϊ________��������ЩCl2��������ͬH2��������__________g������ǡ����ȫ��Ӧ��õ����Ȼ�����������ʵ�����________Ħ��������Ϊ________g���ڱ�״������_______�������������Ȼ�����������490.4 mLˮ�У���������Һ������Ϊ________g����������Һ���ܶ�Ϊ1.01g/cm3�������Һ�����Ϊ_________ L����Һ�����ʵ���Ũ��Ϊ_________mol/L����������Ϊ__________��
��2�����������ʣ��� �ռ��SO3 �� ��Ƭ�� ʯī�� ���� ���� ������ �����ᱵ�����ڵ���ʵ���__________��������ţ���ͬ������������ǿ����ʵ���____________�����ڷǵ���ʵ���__________���Ȳ����ڵ�����ֲ����ڷǵ���ʵ���____________��
��3����״���£�1 ���ˮ���ܽ�448 ���HCl��������Һ���ܶ�Ϊ1.2g/mL������Һ���������������ʵ�����Ũ�ȷֱ���____________��_____________��������1λС����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ��һ�����л�ѧ���������棩 ���ͣ�ѡ����
��Na��Mg��Al��0.4mol�ֱ����100mL��2mol/L�������У�ͬ��ͬѹ�²����������������ǣ�
A��1:1:1 B��2:1:1 C��3��1��1 D��1:2:3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ��һ�����л�ѧ���������棩 ���ͣ�ѡ����
��NA��������ӵ�����������˵����ȷ����( )
A��22.4LCO��CO2�Ļ��������������̼ԭ����һ����NA
B����1molHCl��������Һ������Fe��Ӧ��Fe��ʧȥ�ĵ�������Ϊ2NA
C�����³�ѹ�£�32gO2��32gO3������ԭ��������2NA
D����״���£�11.2LH2O����0.5NA����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ������ʮ��У�߶���ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
25 ��ʱ��ˮ�ĵ���ﵽƽ�⣺H2O H+ + OH-������������ȷ����( )
H+ + OH-������������ȷ����( )
A����ˮ�м�������Na���壬ƽ�������ƣ�c(H+)����
B����ˮ�м���ϡ��ˮ��ƽ�������ƶ���c(OH-)����
C����ˮ�м��������������ƹ��壬c(H+)����Kw���䶯
D����ˮ���ȣ�Kw����pH����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com