
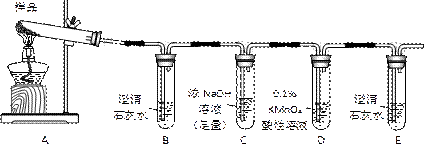




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��װ�â��У�dΪ������cΪ���� |
| B��װ�âڿ������ռ�H2��NH3��CO2��Cl2��HCl��NO2������ |
| C��װ�â���X��ΪCCl4������������NH3��HCl����NH3��HCl������CCl4�����ɷ�ֹ���� |
| D��װ�âܿ����ڸ���ռ�NH3�������ն����NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
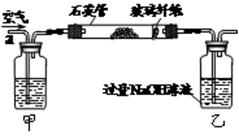
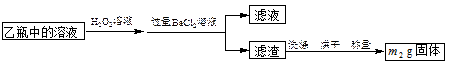

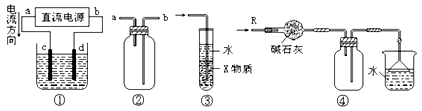
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ����ش��������⣺
����ش��������⣺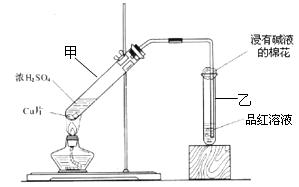
 L��Ba(OH)2��Һ�����ٲ�������ʱ��ǡ������40.00 mL Ba(OH)2��Һ������㣺
L��Ba(OH)2��Һ�����ٲ�������ʱ��ǡ������40.00 mL Ba(OH)2��Һ������㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

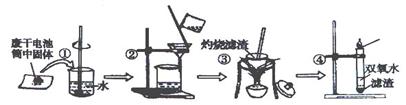
| ʵ��Ŀ�� | ���� | ʵ������ | ���� |
| ����Cl�� | ȡ������Һ���Թ��У� | | ����Cl�� |
| ����NH4�� | ȡ������Һ���Թ��У� | | ����NH4�� |
| ����Zn2�� | ȡ������Һ���Թ��У� | | ����Zn2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
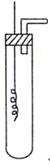
 �����ƶ������Թ�Һ���У�ʹ֮�����ᷴӦ����Ӧ�����ӷ���ʽ�� ��
�����ƶ������Թ�Һ���У�ʹ֮�����ᷴӦ����Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
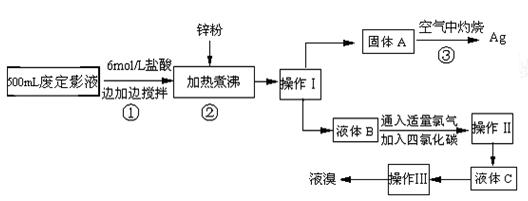
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
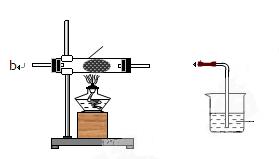
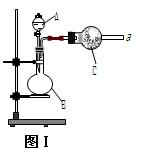
 ��ʵ���й۲�
��ʵ���й۲� ��E���к���ɫ������֣�֤����������_________�ԣ�
��E���к���ɫ������֣�֤����������_________�ԣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
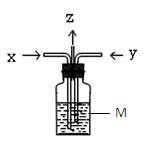 ��
�� | A����MΪBaCl2��Һ����ϴ��ƿ�л����BaCO3��BaSO3���� |
| B����MΪBa(OH)2��Һ��ϴ��ƿ��ֻ�����BaCO3���� |
C����MΪKI������Һ��ϴ��ƿ����Һһ���������ɫ |
| D����MΪˮ����z���ܿ��к���ɫ������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com