
ŃõĘų(O2)ŗĶ³ōŃõ(O3)ŹĒŃõŌŖĖŲµÄĮ½ÖÖĶ¬ĖŲŅģŠĪĢ壬ŅŃÖŖČČ»Æѧ·½³ĢŹ½£ŗ
4Al(s)£«3O2(g)===2Al2O3(s)””¦¤H1 ¢Ł
4Al(s)£«2O3(g)===2Al2O3(s)””¦¤H2 ¢Ś
3O2(g)===2O3(g)””¦¤H3 ¢Ū
Ōņ (””””)
A£®¦¤H1£¦¤H2£½¦¤H3 B£®¦¤H1£«¦¤H2£½¦¤H3
C£®¦¤H2£¦¤H1£½¦¤H3 D£®¦¤H1£«¦¤H2£«¦¤H3£½0
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijČÜŅŗÖŠæÉÄÜŗ¬ÓŠH£«”¢NH ”¢Mg2£«”¢Al3£«”¢Fe3£«”¢CO
”¢Mg2£«”¢Al3£«”¢Fe3£«”¢CO ”¢SO
ӢSO ӢNO
”¢NO ÖŠµÄ¼øÖÖ”£¢ŁČō¼ÓČėŠæĮ££¬²śÉśĪŽÉ«ĪŽĪ¶µÄĘųĢ壻¢ŚČō¼ÓČėNaOHČÜŅŗ£¬²śÉś°×É«³Įµķ£¬ĒŅ²śÉśµÄ³ĮµķĮæÓė¼ÓČėNaOHµÄĪļÖŹµÄĮæÖ®¼äµÄ¹ŲĻµČēĶ¼ĖłŹ¾”£ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
ÖŠµÄ¼øÖÖ”£¢ŁČō¼ÓČėŠæĮ££¬²śÉśĪŽÉ«ĪŽĪ¶µÄĘųĢ壻¢ŚČō¼ÓČėNaOHČÜŅŗ£¬²śÉś°×É«³Įµķ£¬ĒŅ²śÉśµÄ³ĮµķĮæÓė¼ÓČėNaOHµÄĪļÖŹµÄĮæÖ®¼äµÄ¹ŲĻµČēĶ¼ĖłŹ¾”£ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
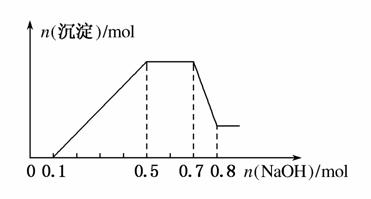
A£®ČÜŅŗÖŠµÄŃōĄė×ÓÖ»ÓŠH£«”¢Mg2£«”¢Al3£«
B£®ČÜŅŗÖŠn(NH )£½0.2 mol
)£½0.2 mol
C£®ČÜŅŗÖŠŅ»¶Ø²»ŗ¬CO £¬æÉÄÜŗ¬ÓŠSO
£¬æÉÄÜŗ¬ÓŠSO ŗĶNO
ŗĶNO
D£®n(H£«):n(Al3£«):n(Mg2£«)£½1:1:1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓŠŅ»Ęæ³ĪĒåµÄČÜŅŗ£¬ĘäÖŠæÉÄÜŗ¬ÓŠNH ”¢K£«”¢Mg2£«”¢Ba2£«”¢Al3£«”¢Fe3£«”¢SO
”¢K£«”¢Mg2£«”¢Ba2£«”¢Al3£«”¢Fe3£«”¢SO ”¢CO
ӢCO ӢNO
”¢NO ŗĶI£”£Č”øĆČÜŅŗ½ųŠŠŅŌĻĀŹµŃé£ŗ
ŗĶI£”£Č”øĆČÜŅŗ½ųŠŠŅŌĻĀŹµŃé£ŗ
(1)ÓĆpHŹŌÖ½¼ģŃ飬±ķĆ÷ČÜŅŗ³ŹĒæĖįŠŌ£»
(2)Č”²æ·ÖČÜŅŗ£¬¼ÓČėÉŁĮæCCl4¼°ŹżµĪŠĀÖʵÄĀČĖ®£¬¾Õńµ“ŗó£¬CCl4³Ź×ĻŗģÉ«£»
(3)ĮķČ”²æ·ÖČÜŅŗ£¬ÖšµĪ¼ÓČėĻ”NaOHČÜŅŗ£¬Ź¹ČÜŅŗ“ÓĖįŠŌÖš½„×Ŗ±äĪŖ¼īŠŌ£¬ŌŚµĪ¼Ó¹ż³ĢÖŠ£¬ČÜŅŗÖŠ¾łĪŽ³ĮµķÉś³É£»
(4)Č”²æ·ÖÉĻŹö¼īŠŌČÜŅŗ¼ÓČČ£¬¼ÓNa2CO3ČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£»
(5)½«(3)µĆµ½µÄ¼īŠŌČÜŅŗ¼ÓČČ£¬ÓŠĘųĢå·Å³ö£¬øĆĘųĢåÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶”£
øł¾ŻÉĻŹöŹµŃéŹĀŹµČ·¶Ø£ŗ ŌŚøĆČÜŅŗÖŠæĻ¶Ø“ęŌŚµÄĄė×ÓŹĒ______________£¬æĻ¶Ø²»“ęŌŚµÄĄė×ÓŹĒ________________________________________________________________________
______________£¬»¹²»ÄÜČ·¶ØŹĒ·ń“ęŌŚµÄĄė×ÓŹĒ________”£ČēŗĪ¼ģŃé²»ÄÜČ·¶ØµÄĄė×ÓŹĒ·ń“ęŌŚ£æ________________________________________________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ē¦Šīµē³ŲµÄ¹¤×÷ŌĄķĪŖ£ŗPb£«PbO2£«2H2SO4===2PbSO4£«2H2O”£ŃŠ¶ĮĻĀĶ¼£¬ĻĀĮŠÅŠ¶Ļ²»ÕżČ·µÄŹĒ(””””)
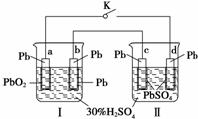
A£®K±ÕŗĻŹ±£¬dµē¼«·“Ó¦Ź½£ŗPbSO4£«2H2O”Ŗ”śPbO2£«4H£«£«SO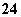 £«2e£
£«2e£
B£®µ±µēĀ·ÖŠ×ŖŅĘ0.2 molµē×ÓŹ±£¬¢ńÖŠĻūŗĵÄH2SO4ĪŖ0.2 mol
C£®K±ÕŗĻŹ±£¬¢ņÖŠSO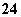 Ļņcµē¼«ĒØŅĘ
Ļņcµē¼«ĒØŅĘ
D£®K±ÕŗĻŅ»¶ĪŹ±¼äŗ󣬢ņæɵ„¶Ą×÷ĪŖŌµē³Ų£¬dµē¼«ĪŖÕż¼«
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
µāŌŚ²»Ķ¬×“Ģ¬ĻĀ£Ø¹ĢĢ¬»ņĘųĢ¬£©ÓėĒāĘų·“Ó¦µÄČČ»Æѧ·½³Ģ
Ź½ČēĻĀĖłŹ¾£ŗ
¢Ł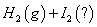 =
=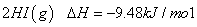
¢Ś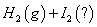 =
=
ĻĀĮŠÅŠ¶ĻÕżČ·µÄŹĒ
A£®¢ŁÖŠµÄ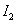 ĪŖ¹ĢĢ¬£¬¢ŚÖŠµÄ
ĪŖ¹ĢĢ¬£¬¢ŚÖŠµÄ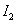 ĪŖĘųĢ¬
ĪŖĘųĢ¬
B£®¢ŚµÄ·“Ó¦Īļ×ÜÄÜĮæ±Č¢ŁµÄ·“Ó¦Īļ×ÜÄÜĮæµĶ
C£®¢ŁµÄ²śĪļ±Č·“Ó¦¢ŚµÄ²śĪļČČĪČ¶ØŠŌøüŗĆ
D£®1mol¹ĢĢ¬µāÉż»ŖŹ±½«ĪüČČ17kJ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖĻĀĮŠø÷·“Ó¦¾łĪŖ·ÅČČ·“Ó¦£¬Ķ¬ĪĀĶ¬Ń¹ĻĀ£¬ĻĀĮŠø÷ČČ»Æѧ·½³ĢŹ½ÖŠµÄ¦¤H×īŠ”µÄŹĒ (””””)
A£®2A(l)£«B(l)===2C(g)””¦¤H1
B£®2A(g)£«B(g)===2C(g)””¦¤H2
C£®2A(g)£«B(g)===2C(l)””¦¤H3
D£®2A(l)£«B(l)===2C(l)””¦¤H4
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÄÜŌ“ŹĒ¹śĆń¾¼Ć·¢Õ¹µÄÖŲŅŖ»ł“”£¬ĪŅ¹śÄæĒ°Ź¹ÓƵÄÄÜŌ“Ö÷ŅŖŹĒ»ÆŹÆČ¼ĮĻ”£
£Ø1£©ŌŚ25 ”ę”¢101 kPaŹ±£¬16 g CH4ĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®Ź±·Å³öµÄČČĮæŹĒ890.31 kJ£¬ŌņCH4Č¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ŹĒ________________________________”£
£Ø2£©ŅŃÖŖ£ŗC£Øs£©£«O2£Øg£©===CO2£Øg£©£»¦¤H£½£437.3 kJ·mol£1
H2£Øg£©£« O2£Øg£©===H2O£Øg£©£»¦¤H£½£285.8 kJ·mol£1
O2£Øg£©===H2O£Øg£©£»¦¤H£½£285.8 kJ·mol£1
CO£Øg£©£« O2£Øg£©===CO2£Øg£©£»¦¤H£½£283.0 kJ·mol£1
O2£Øg£©===CO2£Øg£©£»¦¤H£½£283.0 kJ·mol£1
ŌņĆŗµÄĘų»ÆÖ÷ŅŖ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒ
C£Øs£©£«H2O£Øg£©===CO£Øg£©£«H2£Øg£©£»¦¤H£½________kJ·mol£1”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
0.1 mol”¤L£1 NaHCO3ČÜŅŗµÄpH×ī½Ó½üÓŚ(””””)””””””””””””””””””””””””””””””””””
A£®5.6 B£®7.0 C£®8.4 D£®13.0
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĄąĶʵÄĖ¼Ī¬·½·ØŌŚ»ÆѧѧĻ°ÓėŃŠ¾æÖŠÓŠŹ±»į²śÉś“ķĪó½įĀŪ£¬Ņņ“ĖĄąĶĘµÄ½įĀŪ×īÖÕŅŖ¾¹żŹµ¼łµÄ¼ģŃ飬²ÅÄܾö¶ØĘäÕżČ·Óė·ń£¬ĻĀĮŠ¼øÖÖĄąĶĘ½įĀŪÖŠ£¬“ķĪóµÄŹĒ(””””)
¢ŁÄĘÓėĖ®·“Ӧɜ³ÉNaOHŗĶH2£»ĖłÓŠ½šŹōÓėĖ®·“Ó¦¶¼Éś³É¼īŗĶH2””¢ŚĢśĀ¶ÖĆŌŚæÕĘųÖŠŅ»¶ĪŹ±¼äŗó¾Ķ»įÉśŠā£»ŠŌÖŹøü»īĘƵÄĀĮ²»ÄÜĪČ¶Ø“ęŌŚÓŚæÕĘųÖŠ””¢Ū»ÆŗĻĪļNaClµÄŃęÉ«ĪŖ»ĘÉ«£»Na2CO3µÄŃęÉ«Ņ²ĪŖ»ĘÉ«””¢ÜĆܶČĪŖ1.1 g/cm3ÓėĆܶČĪŖ1.2 g/cm3µÄNaClČÜŅŗµČĢå»ż»ģŗĻ£¬ĖłµĆNaClČÜŅŗµÄĆÜ¶Č½ēÓŚ1.1 g/cm3Óė1.2 g/cm3Ö®¼ä£»Na£KŗĻ½šµÄČŪµćÓ¦½ēÓŚNaŗĶKČŪµćÖ®¼ä
A£®¢Ł¢Ś B£®¢Ł¢Ü
C£®¢Ł¢Ś¢Ū¢Ü D£®¢Ł¢Ś¢Ü
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com