
 ���أ�H2NCONH2�����������л����ʣ���Ҫ������[Fe��H2NCONH2��6]��NO3��3[�����������غ�������]��
���أ�H2NCONH2�����������л����ʣ���Ҫ������[Fe��H2NCONH2��6]��NO3��3[�����������غ�������]�� ��֪�����ط�����Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3���ݴ��жϣ�
��֪�����ط�����Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3���ݴ��жϣ�| m |
| V |
 ��֪�����ط�����Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3��Cԭ�Ӳ�ȡsp2�ӻ���Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����ʴ�Ϊ��sp2��sp3��
��֪�����ط�����Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3��Cԭ�Ӳ�ȡsp2�ӻ���Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����ʴ�Ϊ��sp2��sp3��| 1 |
| 8 |
| 1 |
| 2 |
| 1 |
| 8 |
| 1 |
| 2 |
| ||
| (a��10-10)3 |
| 1.76��1032 |
| aNA |
| 1.76��1032 |
| aNA |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���Թ�Ҫ��Ũ���������ˮϴ�� |
| B������ˮԡ���ȣ�����ֱ�Ӽ��� |
| C����2%��ϡ��ˮ�е���2%����������Һ�����������Һ |
| D������Ũ����ϴȥ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��2Na2O2+2H2O�T4NaOH+O2�� | ||||
B��C+H2O
| ||||
| C��CO2+NH3+H2O�TNH4HCO3 | ||||
| D��2F2+2H2O�T4HF+O2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CH4��NH4+ |
| B��N2O4��C2H4 |
| C��CO2��NO2 |
| D��H2O��CH4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ơ��ƿ�ǣ�ƿ��ð���������� |
| B���ϳɰ�����ͨ������20MPa��50MPaѹǿ�������ԭ�ϵ������� |
| C����H2��I2��g����HI��g��������ɵ�ƽ����ϵ��ѹ����ɫ��dz |
| D����ҵ��ȡ������Na��l��+KCl��l��?NaCl��l��+K��g����ѡȡ���˵��¶ȣ�ʹK��������ӷ�Ӧ������з������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
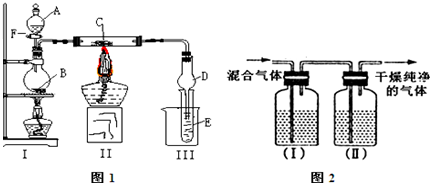
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
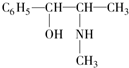
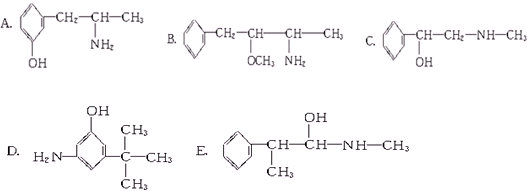
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
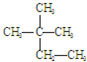 �۰��� ��18O ��
�۰��� ��18O ��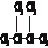 ���� ��16O ��������
���� ��16O ���������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com