
�����ѱ���Ϊ������֮������ġ��������������������ϼ�Ϊ+4�����ǿռ似����������������ҽ���ϲ���ȱ�ٵIJ��ϡ�
��ҵ������������Ҫ�ɷ�FeTiO3���Ʊ������ѵ�һ�ֹ�����������ͼ�����ֲ�����ȥ����
��1������ٷ�Ӧ�Ļ�ѧ����ʽΪ��2FeTiO3 + 6C + 7Cl2 2FeCl3 + 2TiCl4 + 6CO���� ԭ�������� �� ����ÿ����1mol TiCl4ת�� mol���ӡ�
2FeCl3 + 2TiCl4 + 6CO���� ԭ�������� �� ����ÿ����1mol TiCl4ת�� mol���ӡ�
��2������ڷ����TiCl4�ķ�����������TiCl4��FeCl3�� ������ �IJ�ͬ��
��3������ܷ�Ӧ�Ļ�ѧ����ʽΪ______________ __________���÷�Ӧ������н��е�������_________ _____________________��
��4����ɫ��ѧ�ᳫ����ѭ�������������У�������ѭ�������ʳ�Cl2��Mg�⣬����___��
��1��FeTiO3��C�� 7 ��2���е�
��3��2Mg + TiCl4 2MgCl2 + Ti�� ��ֹMg��Ti������ ��4��MgCl2
2MgCl2 + Ti�� ��ֹMg��Ti������ ��4��MgCl2
���������������1�����ݷ�Ӧ�ķ���ʽ��֪��̼Ԫ�صĻ��ϼۺ�FeTiO3��Fe�Ļ��ϼ�Ҳ�����ߵ��ģ����Ի�ԭ����FeTiO3��C�����������������õ�2�����ӣ����Ը��ݷ���ʽ��֪ÿ����1mol TiCl4ת��7mol���ӡ�
��2��TiCl4��FeCl3�ķе㲻ͬ�����Բ���ڷ����TiCl4����������ķ������з��롣
��3��þ�����Ȼ����ڸ��µ������������Ȼ�þ�ͽ����ѣ���Ӧ�Ļ�ѧ����ʽ��2Mg + TiCl4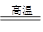 2MgCl2 + Ti�������ѧ�����ȶ������Է�ֹMg��Ti��������
2MgCl2 + Ti�������ѧ�����ȶ������Է�ֹMg��Ti��������
��4����ɫ��ѧ�ᳫ����ѭ���������������У�������ѭ�������ʳ�Cl2��Mg���⣬�����Ȼ�þ�ȡ�
���㣺���������Ʊ����й��жϡ�������ԭ��Ӧ����ƽ�ͼ����Լ���ɫ��ѧ��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⣬�����ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����������Ҫ��ȷ�������ʵ��Ʊ���һ�¼������ͣ�һ����ֻ������Ŀ������ԭ������ȡ��һ�����Լ�ѡȡԭ�����Ʊ������ʣ����е��Ǹ���һ����ҩƷ��������ѡ�ȡ���������Ŀ������ԭ������ȡ����˽�����ʵ����ʣ����Ӧ�ã��𰸾ͻ�ӭ�ж��⡣


 ������������ϵ�д�
������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������/kJ?mol-1 | I1 | I2 | I3 | I4 |
| A | 578 | 1817 | 2745 | 11578 |
| B | 737 | 1451 | 7733 | 10540 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣������ѱ���Ϊ������֮������ġ��������������������ϼ�Ϊ+4�����ǿռ似����������������ҽ���ϲ���ȱ�ٵIJ��ϡ�
��.��ҵ������������Ҫ�ɷ�FeTiO3���Ʊ������ѵ�һ�ֹ�����������ͼ�����ֲ�����ȥ����
��1������ٷ�Ӧ�Ļ�ѧ����ʽΪ��2FeTiO3 + 6C + 7Cl22FeCl3 + 2TiCl4 + 6CO����ԭ���� ��
��2������ڷ����TiCl4�ķ�����������TiCl4��FeCl3 �IJ�ͬ��
��3������ܷ�Ӧ�Ļ�ѧ����ʽΪ________________________________���÷�Ӧ������н��е�������________________________ ______��
��4����ɫ��ѧ�ᳫ����ѭ�������������У�������ѭ�������ʳ�Cl2��Mg�⣬����____________��
II. ��ҵ��Ҳ����TiO2ͨ���������ַ����Ʊ������ѣ�
����һ���Ƚ�TiO2�Ȼ�ΪTiCl4���ٻ�ԭ�õ�Ti��
��5���Ȼ���ӦTiO2 (s)+2Cl2 (g) TiCl4(l) + O2 (g) ���Է�������У��ڷ�Ӧ���м���̼�����ڸ��������·�Ӧ��˳�����С��Դӻ�ѧƽ��ǶȽ��ͣ����Ȼ���Ӧ���м�̼��ԭ�� ��
����������������Ϊ���Һ���TiO2���Ti�����У�̼��Ϊ�������缫��ӦʽΪ��2O2���C4e��=O2����TiO2������������ԭ��
��6�������ĵ缫��ӦʽΪ ��
��7�����������趨��������м���̼���ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ӱ�������ѧ�����꼶ģ�⿼���������� ���ͣ������
�����ѱ���Ϊ������֮������ĵ������������ǿռ似����������������ҽ���ϲ���ȱ�ٵIJ��ϡ�
I����ҵ�����ѿ�ʯ��FeTiO3����FeO��Al2O3��SiO2�����ʣ�����������Ӧ�Ƶã�
���У�����ڷ����ķ�ӦΪ��2H2SO4+FeTiO3=TiOSO4+FeSO4+2H2O��
��1�������ѿ�ʯʱ���Ƿ���Ҫ��ȥ����FeO����_________�����Ҫ������Ҫ����
��2�������ѿ�ʯʱ������Ũ����������Һ��������д���ù����з�����Ӧ�����ӷ���ʽ ��
II��Ϊ�������Դ�������ʣ����ٻ�����Ⱦ���ɽ��ȼ�����ѳ��ͼ״�����ɲ�ҵ��,���ϵ����ͼ��ʾ��
w.w.^w.k.&s.5*u.c.#om
��3��д���������Ȼ����õ����Ȼ��ѵĻ�ѧ����ʽ_________________________��
��4����֪����Mg(s)+Cl2(g)��MgCl2(s)����H��һ641 kJ��mol-1
��1/2Ti(s)+Cl2(g)��1/2TiCl4(l)����H��һ385 kJ��mol-1
��Mg ��TiCl4��Ӧ���Ȼ�ѧ����ʽΪ ��
�÷�Ӧ��������н��е������� ��
��5����������ҵ����,�ϳ�192 t �״�����������ⲹ��H2 t (�������������������ʵ��κ���ʧ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ������ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
�����ѱ���Ϊ������֮������ġ��������������������ϼ�Ϊ+4�����ǿռ似����������������ҽ���ϲ���ȱ�ٵIJ��ϡ�
��ҵ������������Ҫ�ɷ�FeTiO3���Ʊ������ѵ�һ�ֹ�����������ͼ�����ֲ�����ȥ����
��1������ٷ�Ӧ�Ļ�ѧ����ʽΪ��2FeTiO3
+ 6C + 7Cl2 2FeCl3 + 2TiCl4 + 6CO���� ԭ�������� �� ����ÿ����1mol TiCl4ת�� mol���ӡ�
2FeCl3 + 2TiCl4 + 6CO���� ԭ�������� �� ����ÿ����1mol TiCl4ת�� mol���ӡ�
��2������ڷ����TiCl4�ķ�����������TiCl4��FeCl3�� ������ �IJ�ͬ��
��3������ܷ�Ӧ�Ļ�ѧ����ʽΪ______________ __________���÷�Ӧ������н��е�������_________ _____________________��
��4����ɫ��ѧ�ᳫ����ѭ�������������У�������ѭ�������ʳ�Cl2��Mg�⣬����___��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com