
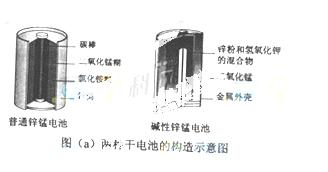
ŠæĆĢµē³Ų£ØĖ׳Ęøɵē³Ų£©ŌŚÉś»īÖŠµÄÓĆĮæŗÜ“ó”£Į½ÖÖŠæĆĢµē³ŲµÄ¹¹ŌģĶ¼ČēĶ¼£Øa£©ĖłŹ¾”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¢ŁĘÕĶØŠæĆĢµē³Ų·ÅµēŹ±·¢ÉśµÄÖ÷ŅŖ·“Ó¦ĪŖ£ŗZn+2NH4Cl+2MnO2=Zn(NH3)2Cl2+2MnOOH”£øƵē³ŲÖŠ£¬øŗ¼«²ÄĮĻÖ÷ŅŖŹĒ___ _________________£¬µē½āÖŹµÄÖ÷ŅŖ³É·ÖŹĒ__________£¬Õż¼«·¢ÉśµÄÖ÷ŅŖ·“Ó¦ŹĒ________________________________________________________”£
_________________£¬µē½āÖŹµÄÖ÷ŅŖ³É·ÖŹĒ__________£¬Õż¼«·¢ÉśµÄÖ÷ŅŖ·“Ó¦ŹĒ________________________________________________________”£
¢ŚÓėĘÕĶØŠæĆĢµē³ŲĻą±Č£¬¼īŠŌŠæĆĢµē³ŲµÄÓÅµć¼°ĘäĄķÓÉŹĒ_______”£
£Ø2£©Ķ¼£Øb£©±ķŹ¾»ŲŹÕĄūÓĆ·Ļ¾ÉĘÕĶØŠæĆĢµē³ŲµÄŅ»ÖÖ¹¤Ņգز»æ¼ĀĒ·Ļ¾Éµē³ŲÖŠŹµ¼Ź“ęŌŚµÄÉŁĮæĘäĖū½šŹō£©”£
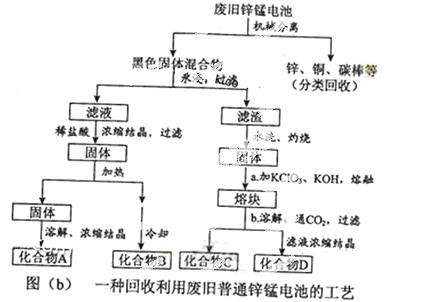
¢ŁĶ¼£Øb£©ÖŠ²śĪļµÄ»ÆѧŹ½·Ö±šĪŖA_______£¬B________”£
¢Ś²Ł×÷aÖŠµĆµ½ČŪæéµÄÖ÷ŅŖ³É·ÖŹĒK2MnO4”£²Ł×÷bÖŠ£¬ĀĢÉ«µÄK2MnO4ČÜŅŗ·“Ó¦ŗóÉś³É×ĻÉ«ČÜŅŗŗĶŅ»ÖÖŗŚŗÖÉ«¹ĢĢ壬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_______”£
¢Ū²ÉÓƶčŠŌµē¼«µē½āK2MnO4ČÜŅŗŅ²ÄܵƵ½»ÆŗĻĪļD£¬ŌņŅõ¼«“¦µĆµ½µÄÖ÷ŅŖĪļÖŹŹĒ____”££ØĢī»ÆѧŹ½£©
 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(1)ŹµŃéŹŅÖŠ³£ÓĆĄ“ÖʱøĀČĘųµÄ»Æѧ·½³ĢŹ½ĪŖ  ”£
ӣ
(2)ŹµŃéŹŅÖŠæÉÓĆĀČ·Ā(CHCl3)ÓėĖ«ŃõĖ®Ö±½Ó·“Ó¦Öʱø¹āĘų£¬Ęä·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓėĖ÷¶ūĪ¬ÖĘ¼ī·ØĻą±Č£¬ŗīµĀ°ńÖĘ¼ī·Ø×īĶ»³öµÄÓŵćŹĒ
A.ŌĮĻĄūÓĆĀŹøß B.Éč±øÉŁ
C.Ń»·ĄūÓƵÄĪļÖŹ¶ą D.ŌĮĻŅ×µĆ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓÉČŪŃĪµē½ā·Ø»ńµĆµÄ“ÖĀĮŗ¬ÓŠŅ»¶ØĮæµÄ½šŹōÄĘŗĶĒāĘų£¬ÕāŠ©ŌÓÖŹæɲÉÓĆ“µĘų¾«Į¶·Ø³żČ„£¬²śÉśµÄĪ²Ęų¾“¦ĄķŗóæÉÓĆøֲĶĘĀĮ”£¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ

(×¢£ŗNaClČŪµćĪŖ801 ”ę£»AlCl3ŌŚ181 ”ęÉż»Ŗ)
(1)¾«Į¶Ē°£¬ŠčĒå³żŪįŪö±ķĆęµÄŃõ»ÆĢśŗĶŹÆӢɰ£¬·ĄÖ¹¾«Į¶Ź±ĖüĆĒ·Ö±šÓėĀĮ·¢ÉśÖĆ»»·“Ó¦²śÉśŠĀµÄŌÓÖŹ£¬Ļą¹ŲµÄ»Æѧ·½³ĢŹ½ĪŖ¢Ł_____________ŗĶ
¢Ś___________________”£
(2)½«Cl2Į¬ŠųĶØČėŪįŪöÖŠµÄ“ÖĀĮČŪĢ壬ŌÓÖŹĖęĘųÅŻÉĻø”³żČ„”£ĘųÅŻµÄÖ÷ŅŖ³É·Ö³żCl2Ķā»¹ŗ¬ÓŠ_____________£»
¹ĢĢ¬ŌÓÖŹÕ³ø½ÓŚĘųÅŻÉĻ£¬ŌŚČŪĢå±ķĆęŠĪ³Éø”Ōü£¬ø”ŌüÖŠæĻ¶Ø“ęŌŚ________________”£
(3)ŌŚÓĆ·Ļ¼īŅŗ“¦ĄķAµÄ¹ż³ĢÖŠ£¬Ėł·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_____________”£
(4)øֲĶĘĀĮŗ󣬱ķĆęŠĪ³ÉµÄÖĀĆÜŃõ»ÆĀĮĤÄÜ·ĄÖ¹øÖ²ÄøÆŹ“£¬ĘäŌŅņŹĒ____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Į×æóŹÆÖ÷ŅŖŅŌĮ×ĖįøĘ”²Ca3(PO4)2·H2O”³ŗĶĮ×»ŅŹÆ”²Ca5(OH)(PO4)3”³µČŠĪŹ½“ęŌŚ”£Ķ¼(a)ĪŖÄæĒ°¹ś¼ŹÉĻĮ×æóŹÆĄūÓƵēóÖĀĒéæö£¬ĘäÖŠŹŖ·ØĮ×ĖįŹĒÖøĮ×æóŹÆÓĆ¹żĮæĮņĖį·Ö½āÖʱøĮ×Ėį”£Ķ¼(b)ŹĒČČ·ØĮ×ĖįÉś²ś¹żø÷ÖŠÓÉĮ×»ŅŹÆÖʵ„ÖŹĮ×µÄĮ÷³Ģ”£
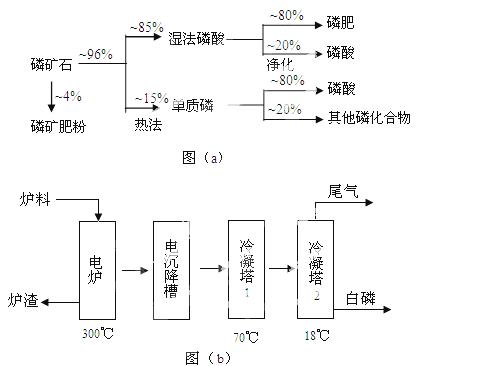
²æ·ÖĪļÖŹµÄĻą¹ŲŠŌÖŹČēĻĀ£ŗ
| ČŪµć/”ę | ·Šµć/”ę | ±ø×¢ | |
| °×Į× | 44 | 280.5 | |
| PH3 | -133.8 | -87.8 | ÄŃČÜÓŚĖ®£¬¾ßÓŠ»¹ŌŠŌ |
| SiF4 | -90 | -86 | Ņ×Ė®½ā |
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŹĄ½ēÉĻĮ×æóŹÆ×īÖ÷ŅŖµÄÓĆĶ¾ŹĒÉś²śŗ¬Į×·ŹĮĻ£¬Ō¼Õ¼Į×æóŹÆŹ¹ÓĆĮæµÄ ℅”£
£Ø2£©ŅŌĮ×»ŅŹÆĪŖŌĮĻ£¬ŹŖ·ØĮ×Ėį¹ż³ĢÖŠCa5F(PO4)3·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ  ”£ĻÖÓŠ1¶ÖÕŪŗĻŗ¬ÓŠĪåŃõ»Æ¶žĮ×Ō¼30%µÄĮ×»ŅŹÆ£¬×ī¶ąæÉÖʵĆ85℅µÄÉĢĘ·
”£ĻÖÓŠ1¶ÖÕŪŗĻŗ¬ÓŠĪåŃõ»Æ¶žĮ×Ō¼30%µÄĮ×»ŅŹÆ£¬×ī¶ąæÉÖʵĆ85℅µÄÉĢĘ· Į×Ėį ¶Ö”£
Į×Ėį ¶Ö”£
£Ø3£©ČēĶ¼(b)ĖłŹ¾£¬ČČ·ØÉś²śĮ×ĖįµÄµŚŅ»²½ŹĒ½«¶žŃõ»Æ¹č”¢¹żĮæ½¹ĢæÓėĮ×»ŅŹÆ»ģŗĻ£¬øßĪĀ·“Ӧɜ³É°×Į×”£ĀÆŌüµÄÖ÷ŅŖ³É·ÖŹĒ£ŗ (Ģī»ÆѧŹ½)ĄäÄżĖž1µÄÖ÷ŅŖ³Į»żĪļŹĒ£ŗ ĄäÄżĖž2µÄÖ÷ŅŖ³Į»żĪļŹĒ£ŗ
£Ø4£©Ī²ĘųÖŠÖ÷ŅŖŗ¬ÓŠ £¬»¹ŗ¬ÓŠÉŁĮæPH3”¢H2SŗĶHFµČ£¬½«Ī² ĘųĻČĶØČė“æ¼īČÜŅŗ£¬
ĘųĻČĶØČė“æ¼īČÜŅŗ£¬ æɳżČ„
æɳżČ„ 
ŌŁĶØČė“ĪĀČĖįÄĘČÜŅŗ£¬æɳżČ„ (¾łĢī»ÆѧŹ½)
£Ø5£©Ļą±ČÓŚŹŖ·ØĮ×Ėį£¬ČČ·ØĮ×Ėį¹¤ŅÕø“ŌÓ£¬ÄÜŗÄøߣ¬µ«ÓŵćŹĒ£ŗ  ”£
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹¤ŅµÉĻÉś²śĮņĖįµÄĮ÷³ĢĶ¼ČēĻĀ£ŗ

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŌēĘŚÉś²śĮņĖįŅŌ»ĘĢśæóĪŖŌĮĻ£¬µ«ĻÖŌŚ¹¤³§Éś²śĮņĖįŅŌĮņ»ĘĪŖŌĮĻ£¬ĄķÓÉŹĒ________________________________________________________________________”£
(2)ŌŚĘųĢå½ųČė“߻Ʒ“Ó¦ŹŅĒ°Šč¾»»ÆµÄŌŅņŹĒ_________________________ ________
________
_____________________ ___________________________________________________ӣ
___________________________________________________ӣ
(3)ŌŚ“߻Ʒ“Ó¦ŹŅÖŠĶس£Ź¹ÓĆ³£Ń¹£¬ŌŚ“ĖĢõ¼žĻĀSO2µÄ×Ŗ»ÆĀŹĪŖ90%”£µ«ŹĒ²æ·Ö·¢“ļ¹ś¼Ņ²ÉČ”øßŃ¹Ģõ¼žĻĀÖĘČ”SO3£¬²ÉČ”¼ÓŃ¹“ėŹ©µÄÄæµÄ³żĮĖ¼Óæģ·“Ó¦ĖŁĀŹĶā£¬»¹æÉŅŌ____________________________£¬“Ó¶ųĢįøßÉś²śŠ§ĀŹ”£
(4)¹¤ŅµÉś²śÖŠ³£ÓĆ°±—Ėį·Ø½ųŠŠĪ²ĘųĶŃĮņ£¬ŅŌ“ļµ½Ļū³żĪŪČ¾£¬·ĻĪļĄūÓƵÄÄæµÄ”£ÓĆ»Æѧ·½³ĢŹ½±ķŹ¾Ęä·“Ó¦ŌĄķ£ŗ____________________________________________________
________________________________________________________________________
________________________________________________________________________ӣ
(5)³żĮņĖį¹¤ŅµĶā£¬»¹ÓŠŠķ¶ą¹¤ŅµÉś²ś”£ĻĀĮŠĻą¹ŲµÄ¹¤ŅµÉś²śĮ÷³ĢÖŠÕżČ·µÄŹĒ________”£
A£®ŗ£Ė®Ģįäå£ŗŗ£Ė®ÅØĖõ
 äåÕōĘų
äåÕōĘų
 Ņŗäå
Ņŗäå
B£®ŗ£Ė®ĢįĆ¾£ŗŗ£Ģ²±“æĒ
 ŹÆ»ŅĖ®
ŹÆ»ŅĖ®
 MgO
MgO Ć¾
Ć¾
C£®¹¤ŅµÖĘĻõĖį£ŗæÕĘų NO2
NO2 ĻõĖį”Ŗ”śĪ²Ęų“¦Ąķ
ĻõĖį”Ŗ”śĪ²Ęų“¦Ąķ
D£®¹¤ŅµŗĻ³É°±£ŗĢģČ»Ęų ĒāĘų
ĒāĘų NH3”¢H2”¢N2
NH3”¢H2”¢N2 °±
°±
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻÖÓŠ¼ø×éĪļÖŹµÄČŪµć( ”ę)Źż¾Ż£ŗ
| A×é | B×é | C×é | D×é |
| ½šøÕŹÆ£ŗ3 550 ”ę | Li£ŗ181 ”ę | HF£ŗ£83 ”ę | NaCl£ŗ801 ”ę |
| ¹č¾§Ģå£ŗ1 410 ”ę | Na£ŗ98 ”ę | HCl£ŗ£115 ”ę | KCl£ŗ776 ”ę |
| Åš¾§Ģå£ŗ2 300 ”ę | K£ŗ64 ”ę | HBr£ŗ£89 ”ę | RbCl£ŗ718 ”ę |
| ¶žŃõ»Æ¹č£ŗ1 723 ”ę | Rb£ŗ39 ”ę | HI£ŗ£51 ”ę | CsCl£ŗ645 ”ę |
¾Ż“Ė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)A×éŹōÓŚ________¾§Ģ壬ĘäČŪ»ÆŹ±æĖ·žµÄĪ¢Į£¼äµÄ×÷ÓĆĮ¦ŹĒ____________________”£
(2)B×龧Ģå¹²Ķ¬µÄĪļĄķŠŌÖŹŹĒ________(ĢīŠņŗÅ)”£
¢ŁÓŠ½šŹō¹āŌ󔔢Śµ¼µēŠŌ””¢Ūµ¼ČČŠŌ””¢ÜŃÓÕ¹ŠŌ
(3)C×éÖŠHFČŪµć·“³£ŹĒÓÉÓŚ_________________”£
(4)D×龧ĢåæÉÄܾßÓŠµÄŠŌÖŹŹĒ________(ĢīŠņŗÅ)”£
¢ŁÓ²¶ČŠ”””¢ŚĖ®ČÜŅŗÄܵ¼µē””¢Ū¹ĢĢåÄܵ¼µē””¢ÜČŪȌדĢ¬Äܵ¼µē
(5)D×龧ĢåµÄČŪµćÓÉøßµ½µĶµÄĖ³ŠņĪŖNaCl>KCl>RbCl>CsCl£¬ĘäŌŅņĪŖ___________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ō×ÓŠņŹżÓÉŠ”µ½“óÅÅĮŠµÄĖÄÖÖ¶ĢÖÜĘŚŌŖĖŲX”¢Y”¢Z”¢W£¬ĘäÖŠX”¢Z”¢WÓėĒāŌŖĖŲæÉ×é³ÉXH3”¢H2ZŗĶHW¹²¼Ū»ÆŗĻĪļ£»YÓėŃõŌŖĖŲæÉ×é³ÉY2OŗĶY2O2Ąė×Ó»ÆŗĻĪļ”£
(1)Š“³öY2O2µÄµē×ÓŹ½£ŗ_____________________£¬
ĘäÖŠŗ¬ÓŠµÄ»Æѧ¼üŹĒ______________£¬ŹōÓŚ¾§ĢåĄąŠĶĪŖ________”£
(2)ÓƵē×ÓŹ½±ķŹ¾Y2OµÄŠĪ³É¹ż³Ģ______________________”£
(3)X”¢Z”¢WČżÖÖŌŖĖŲµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļÖŠ£¬Ļ”ČÜŅŗŃõ»ÆŠŌ×īĒæµÄŹĒ________(Ģī»ÆѧŹ½)”£
(4)XH3”¢H2ZŗĶHWČżÖÖ»ÆŗĻĪļ£¬Ęäæռ乹ŠĶ·Ö±šĪŖ________”¢________”¢________”£ĘäÖŠŅ»ÖÖÓėĮķĶāĮ½ÖÖ¶¼ÄÜ·“Ó¦µÄŹĒ______________(Ģī»ÆѧŹ½)£¬ĘäÉś³ÉĪļ¾§ĢåĄąŠĶ·Ö±šŹōÓŚ________”¢________”£
(5)ÓÉX”¢W×é³ÉµÄ»ÆŗĻĪļ·Ö×ÓÖŠ£¬X”¢WŌ×ÓµÄ×īĶā²ć¾ł“ļµ½8µē×ÓĪČ¶Ø½į¹¹£¬øĆ»ÆŗĻĪļÓöĖ®æÉÉś³ÉŅ»ÖÖ¾ßÓŠĘư׊ŌµÄ»ÆŗĻĪļ£¬ŹŌŠ“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½__________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓÉCO”¢H2ŗĶO2×é³ÉµÄ»ģŗĻĘųĢå60 mL£¬ŌŚŅ»¶ØĢõ¼žĻĀĒ”ŗĆĶźČ«·“Ó¦£¬²āµĆÉś³ÉĪļŌŚ101 kPa”¢120”ęĻĀ¶ŌæÕĘųµÄĻą¶ŌĆܶČĪŖ1.293£¬ŌņŌ»ģŗĻĘųĢåÖŠH2ĖłÕ¼µÄĢå»ż·ÖŹżĪŖ £Ø £©
A£®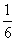 B£®
B£®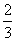 C£®
C£®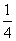 D£®
D£®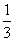
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com