
ijÊÔ¼Á³§ÓẲø(º¬ÔÓÖÊÍ)ºÍÏơËá(º¬ÔÓÖÊFe3+·´Ó¦ÖÆÈ¡ÏơËá̉ø.²½ÖèÈçÏÂ:
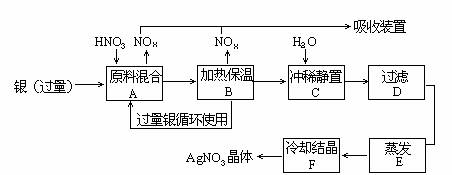
̉À¾ƯÉÏÊö²½Öè,Íê³ÉÏÂÁĐ̀î¿Ơ:
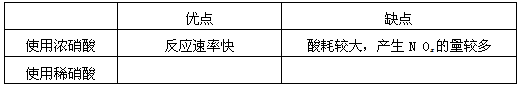
(1)ÈܽẩøµÄÏơËáÓ¦¸ĂÓĂ___________ÏơËá(̀îŨ»̣Ï¡).Ộ̉ÊÇ___________.
(a)¼ơÉÙ¹ư³̀ÖĐ²úÉúNOxµÄÁ¿___________
(b)¼ơÉÙÔÁÏ̉øµÄÏûºÄÁ¿____________
(c)½ÚÊ¡ÏơËáÎïÖʵÄÁ¿___________
(2)²½ÖèB¼ÓÈȱ£ÎµÄ×÷ÓĂÊÇ___________.
(a)ÓĐÀûÓÚ¼Ó¿́·´Ó¦ËÙ¶È
(b)ÓĐÀûÓÚδ·´Ó¦µÄÏơËá»Ó·¢
(c)ÓĐÀûÓÚÏơËá³ä·Ö·´Ó¦,½µµÍÈÜ̉ºÖĐ[H+]
(3)²½ÖèCÊÇΪÁ˳ưÈ¥Fe3+¡¢Cu2+µÈÔÓÖÊ.³åÏ¡¾²ÖĂʱ·¢ÉúµÄ»¯Ñ§·´Ó¦ÊÇ___________;
(a)ÖĂ»»·´Ó¦(b)Ë®½â·´Ó¦(c)Ñơ»¯¨D»¹Ô·´Ó¦²úÉúµÄ³ÁµíÎﻯѧʽ:___________.

| Ä꼶 | ¸ßÖĐ¿Î³̀ | Ä꼶 | ³ơÖĐ¿Î³̀ |
| ¸ß̉» | ¸ß̉»Ăâ·Ñ¿Î³̀ÍƼö£¡ | ³ở» | ³ở»Ăâ·Ñ¿Î³̀ÍƼö£¡ |
| ¸ß¶₫ | ¸ß¶₫Ăâ·Ñ¿Î³̀ÍƼö£¡ | ³ơ¶₫ | ³ơ¶₫Ăâ·Ñ¿Î³̀ÍƼö£¡ |
| ¸ßÈư | ¸ßÈưĂâ·Ñ¿Î³̀ÍƼö£¡ | ³ơÈư | ³ơÈưĂâ·Ñ¿Î³̀ÍƼö£¡ |
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£ºÎïÀí½̀ÑĐỂ ̀âĐÍ£º058
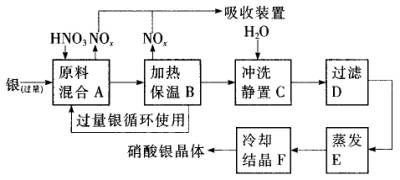
¸ù¾ƯÉÏÊö²½Ö裬Íê³ÉÏÂÁĐ̀î¿Ơ£º
£¨1£©ÈܽẩøµÄÏơËáÓ¦¸ĂÓĂ________£῭î¡°Ï¡¡±»̣¡°Å¨¡±£©£¬Ộ̉ÊÇ________£®
¡¡¡¡£¨a£©¼ơÉÙ¹ư³̀ÖĐ²úÉúNOxµÄÁ¿
¡¡¡¡£¨b£©¼ơÉÙÔÁÏ̉øµÄÏûºÄÁ¿
£¨c£©½ÚÊ¡ÏơËáµÄÎïÖʵÄÁ¿
£¨2£©²½ÖèB¼ÓÈȱ£ÎµÄ×÷ÓĂÊÇ________£®
¡¡¡¡£¨a£©ÓĐÀûÓÚ¼Ó¿́·´Ó¦ËÙÂÊ
¡¡¡¡£¨b£©ÓĐÀûÓÚδ·´Ó¦µÄÏơËá»Ó·¢
¡¡¡¡£¨c£©ÓĐÀûÓÚÏơËá³ä·Ö·´Ó¦£¬½µµÍÈÜ̉ºÖĐ[H+]
£¨3£©²½ÖèCÊÇΪÁ˳ưÈ¥Fe3+¡¢Cu2+µÈÔÓÖÊ£¬³åÏ´¡¢¾²ÖĂ¿É·¢ÉúµÄ»¯Ñ§·´Ó¦ÊÇ________£®
¡¡¡¡£¨a£©ÖĂ»»·´Ó¦
¡¡¡¡£¨b£©Ë®½â·´Ó¦
¡¡¡¡£¨c£©Ñơ»¯»¹Ô·´Ó¦
¡¡¡¡²úÉúµÄ³ÁµíÎﻯѧʽ________£®
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º2011-2012ѧÄê°²»ƠÊ¡¸ßÈưµÚ̉»Ñ§ÆÚÆÚÖĐ¿¼ÊÔ»¯Ñ§ÊÔ¾í ̀âĐÍ£ºÊµÑé̀â
£¨14·Ö£¬Ă¿¿Ơ2·Ö£©Ä³ÊÔ¼Á³§ÓẲø£¨º¬ÔÓÖÊÍ£©ºÍÏơËᣨº¬Fe3+£©·´Ó¦ÖÆÈ¡ÏơËá̉ø£¬²½ÖèÈçÏ£º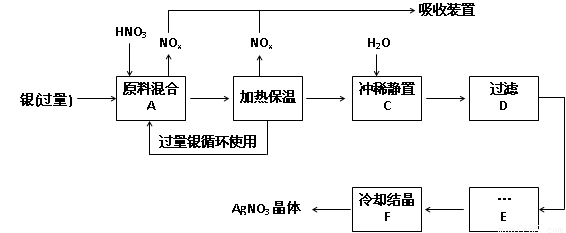
£¨1£©¹¤̉µÉÏ̉»°ăÑ¡ÓĂÖеÈŨ¶ÈµÄÏơËáºÍ̉ø·´Ó¦À´ÖÆÈ¡ÏơËá̉ø¡£ÇëÔÚϱí¿Ơ¸ñ´¦̀î¿Ơ¡£
|
|
Óŵă |
ȱµă |
|
ʹÓĂŨÏơËá |
·´Ó¦ËÙÂÊ¿́ |
ËáºÄ½Ï´ó£¬²úÉúNOxµÄÁ¿½Ï¶à |
|
ʹÓĂÏ¡ÏơËá |
|
|
£¨2£©²½ÖèB¼ÓÈȱ£ÎµÄ×÷ÓĂÊÇ £º
a£® ÓĐÀûÓÚ¼Ó¿́·´Ó¦ËÙÂÊ
b£®ÓĐÀûÓÚδ·´Ó¦µÄÏơËá»Ó·¢
c£®ÓĐÀûÓÚÏơËá³ä·Ö·´Ó¦£¬½µµÍÈÜ̉ºÖĐH+µÄŨ¶È
£¨3£©²½ÖèCÊÇΪÁ˳ưÈ¥Fe3+¡¢Cu2+µÈÔÓÖÊ£¬³åϡʱ²úÉú³ÁµíµÄỘ̉ÊÇ £»
£¨4£©²½ÖèCÖĐ¼ÓË®µÄÁ¿Ó¦¸ĂÊÊÁ¿£¬Èô¼ÓÈë¹ư¶àµÄË®£¬¶ÔºóĐø²½ÖèỐ³ÉµÄ²»Á¼Ó°ḮÊÇ£º
£»
£¨5£©²½ÖèE½øĐĐµÄ²Ù×÷ÊÇ ¡£
£¨6£©ÖÆµĂµÄÏơËá̉øÖĐº¬ÓĐÉÙÁ¿ÏơËáÍ£¬Í¨³£³ưÈ¥ÏơËá͵ķ½·¨ÊÇÔÚ²½ÖèE֮ǰ¼ÓÊÊÁ¿ĐÂÖƵÄAg2O£¬Ê¹Cu2+ת»¯ÎªCu(OH)2³Áµí£¬·´Ó¦ºó¹ưÂ˳ưÈ¥¡£¸Ă·´Ó¦µÄ»¯Ñ§·½³̀ʽΪ£º ¡£
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º0112 ÆÚÖĐ̀â ̀âĐÍ£º̀î¿Ờâ
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º
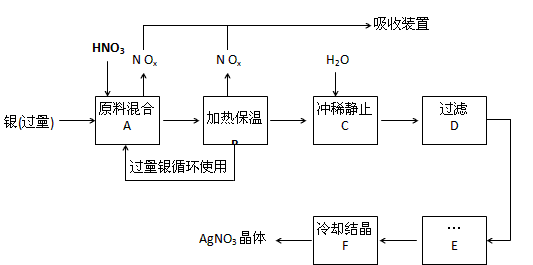
£¨12·Ö£©Ä³ÊÔ¼Á³§ÓẲø£¨º¬ÔÓÖÊÍ£©ºÍÏơËᣨº¬Fe3+£©·´Ó¦ÖÆÈ¡ÏơËá̉ø£¬²½ÖèÈçÏ£º
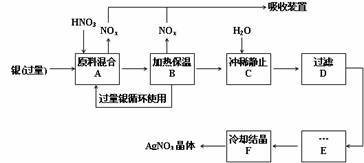
£¨1£©¹¤̉µÉÏ̉»°ăÑ¡ÓĂÖеÈŨ¶ÈµÄÏơËáºÍ̉ø·´Ó¦À´ÖÆÈ¡ÏơËá̉ø¡£ÇëÔÚϱí¿Ơ¸ñ´¦̀î¿Ơ¡£
| Óŵă | ȱµă |
ʹÓĂŨÏơËá | ·´Ó¦ËÙÂÊ¿́ | ËáºÄ½Ï´ó£¬²úÉúNOxµÄÁ¿½Ï¶à |
ʹÓĂÏ¡ÏơËá |
|
|
£¨2£©²½ÖèB¼ÓÈȱ£ÎµÄ×÷ÓĂÊÇ £º
a£®ÓĐÀûÓÚ¼Ó¿́·´Ó¦ËÙÂÊ
b£®ÓĐÀûÓÚδ·´Ó¦µÄÏơËá»Ó·¢
c£®ÓĐÀûÓÚÏơËá³ä·Ö·´Ó¦£¬½µµÍÈÜ̉ºÖĐH+µÄŨ¶È
£¨3£©²½ÖèCÊÇΪÁ˳ưÈ¥Fe3+¡¢Cu2+µÈÔÓÖÊ£¬³åϡʱ²úÉú³ÁµíµÄỘ̉ÊÇ £»
£¨4£©²½ÖèCÖĐ¼ÓË®µÄÁ¿Ó¦¸ĂÊÊÁ¿£¬Èô¼ÓÈë¹ư¶àµÄË®£¬¶ÔºóĐø²½ÖèỐ³ÉµÄ²»Á¼Ó°ḮÊÇ£º £»
£¨5£©²½ÖèE½øĐĐµÄ²Ù×÷ÊÇ ¡£
£¨6£©ÖÆµĂµÄÏơËá̉øÖĐº¬ÓĐÉÙÁ¿ÏơËáÍ£¬Í¨³£³ưÈ¥ÏơËá͵ķ½·¨ÊÇÔÚ²½ÖèE֮ǰ¼ÓÊÊÁ¿ĐÂÖƵÄAg2O£¬Ê¹Cu2+ת»¯ÎªCu(OH)2³Áµí£¬·´Ó¦ºó¹ưÂ˳ưÈ¥¡£¸Ă·´Ó¦µÄ»¯Ñ§·½³̀ʽΪ£º ¡£
²é¿´´đ°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º ̀âĐÍ£º
£¨14·Ö£¬Ă¿¿Ơ2·Ö£©Ä³ÊÔ¼Á³§ÓẲø£¨º¬ÔÓÖÊÍ£©ºÍÏơËᣨº¬Fe3+£©·´Ó¦ÖÆÈ¡ÏơËá̉ø£¬²½ÖèÈçÏ£º
£¨1£©¹¤̉µÉÏ̉»°ăÑ¡ÓĂÖеÈŨ¶ÈµÄÏơËáºÍ̉ø·´Ó¦À´ÖÆÈ¡ÏơËá̉ø¡£ÇëÔÚϱí¿Ơ¸ñ´¦̀î¿Ơ¡£
|
| Óŵă | ȱµă |
| ʹÓĂŨÏơËá | ·´Ó¦ËÙÂÊ¿́ | ËáºÄ½Ï´ó£¬²úÉúNOxµÄÁ¿½Ï¶à |
| ʹÓĂÏ¡ÏơËá |
|
|
£¨2£©²½ÖèB¼ÓÈȱ£ÎµÄ×÷ÓĂÊÇ £º
a£® ÓĐÀûÓÚ¼Ó¿́·´Ó¦ËÙÂÊ
b£®ÓĐÀûÓÚδ·´Ó¦µÄÏơËá»Ó·¢
c£®ÓĐÀûÓÚÏơËá³ä·Ö·´Ó¦£¬½µµÍÈÜ̉ºÖĐH+µÄŨ¶È
£¨3£©²½ÖèCÊÇΪÁ˳ưÈ¥Fe3+¡¢Cu2+µÈÔÓÖÊ£¬³åϡʱ²úÉú³ÁµíµÄỘ̉ÊÇ £»
£¨4£©²½ÖèCÖĐ¼ÓË®µÄÁ¿Ó¦¸ĂÊÊÁ¿£¬Èô¼ÓÈë¹ư¶àµÄË®£¬¶ÔºóĐø²½ÖèỐ³ÉµÄ²»Á¼Ó°ḮÊÇ£º
£»
£¨5£©²½ÖèE½øĐĐµÄ²Ù×÷ÊÇ ¡£
£¨6£©ÖÆµĂµÄÏơËá̉øÖĐº¬ÓĐÉÙÁ¿ÏơËáÍ£¬Í¨³£³ưÈ¥ÏơËá͵ķ½·¨ÊÇÔÚ²½ÖèE֮ǰ¼ÓÊÊÁ¿ĐÂÖƵÄAg2O£¬Ê¹Cu2+ת»¯ÎªCu(OH)2³Áµí£¬·´Ó¦ºó¹ưÂ˳ưÈ¥¡£¸Ă·´Ó¦µÄ»¯Ñ§·½³̀ʽΪ£º ¡£
²é¿´´đ°¸ºÍ½âÎö>>
°Ù¶ÈÖÂĐÅ - Á·Ï°²áÁбí - ÊỒâÁбí
º₫±±Ê¡»¥ÁªÍøÎ¥·¨ºÍ²»Á¼ĐÅÏ¢¾Ù±¨Æ½̀¨ | ÍøÉÏÓĐº¦ĐÅÏ¢¾Ù±¨×¨Çø | µçĐÅƠ©Æ¾Ù±¨×¨Çø | ÉæÀúÊ·ĐéÎ̃Ö÷̉åÓĐº¦ĐÅÏ¢¾Ù±¨×¨Çø | ÉæÆóÇÖȨ¾Ù±¨×¨Çø
Î¥·¨ºÍ²»Á¼ĐÅÏ¢¾Ù±¨µç»°£º027-86699610 ¾Ù±¨ÓÊÏ䣺58377363@163.com