
�������ڲ�ͬ�����¿��Ա������ɲ�ͬ���ʡ���������ش����⣺
��֪RCOOH��CH2===CH2��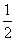 O2
O2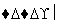 RCOOCH===CH2��H2O
RCOOCH===CH2��H2O
(1)�������ھƻ�ø�����������л���A��A��B��C��D��E���ת����ϵ��ͼ��ʾ��
��B��ʯ�ͻ�ѧ��ҵ����Ҫ�Ļ���ԭ�ϣ�д��A�D��B�Ļ�ѧ����ʽ��
________________________________________________________________________
________________________________________________________________________��
��D�Ľṹ��ʽΪ
________________________________________________________________________��
(2)��������һ�������»��ɱ�����ΪX��Y(Y��A����Է���������ͬ)��X�ɴ�������Y��Ҳ����H2��Ӧ����Z��X��Y�Ľṹ����һ����ͬ�Ĺ�������____________������˹��������õ��Լ���________________________________________��
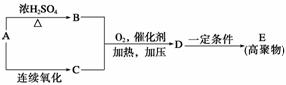
(3)F�����弡��ϸ���е���������ȱ�������½������������IJ��F��G��H���ת����ϵ��F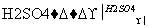 G
G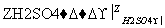 H��H��(1)�е�D��Ϊͬ���칹�塣
H��H��(1)�е�D��Ϊͬ���칹�塣
��G�����Է����ķ�Ӧ��________(�����)
��.�ӳɷ�Ӧ ��.ˮ�ⷴӦ
��.������Ӧ ��.��ȥ��Ӧ
��.��ԭ��Ӧ
�ڱ����漰�������л����У���F�����Ժ��������Ȼ��(������һ��)����ȫȼ������CO2��H2O�����ʵ����������(д���ṹ��ʽ)____________________��
(1)��C2H5OH CH2CH2����H2O
CH2CH2����H2O
��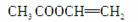
(2)��CHO��������Һ(������������ͭ����Һ)
(3)�٢�
��HOCH2(CHOH)4CHO��HCHO��CH3COOH
������(1)�������ھƻ�ø�����������Ҵ���CO2������A���Ҵ��������ʵ�ת����ϵ��֪BΪ ��CΪCH3COOH���������Ϣ��֪B��C��Ӧ���ɵ�DΪ
��CΪCH3COOH���������Ϣ��֪B��C��Ӧ���ɵ�DΪ
 ��
��
(2)��X������Y���ֿ���H2��Ӧ����Z������֪XΪȩ��YΪ�ᣬZΪ����������Y��CH3CH2OH����Է���������ͬ����֪YΪ���ᣬXΪ��ȩ��ZΪ�״����ڼ����д��ڡ�CHO��
(3)ZΪ�״�����G��Z�D��H��H�� ��Ϊͬ���칹�����֪GΪ
��Ϊͬ���칹�����֪GΪ ��HΪ
��HΪ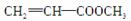 ����ת����ϵ��֪FΪ
����ת����ϵ��֪FΪ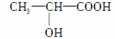 ��
��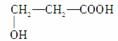 ��������ѧ��ʶ��֪FΪǰ������ṹ������ʽΪC3H6O3���ڷ���Ҫ����л�������������ʽΪCH2O��
��������ѧ��ʶ��֪FΪǰ������ṹ������ʽΪC3H6O3���ڷ���Ҫ����л�������������ʽΪCH2O��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����(����)
A���������֮�͵��������ɸ߾��������
B������Ϊһ������ʱ�����巢���Ӿ۷�Ӧ
C�����۷�Ӧ�ĵ�����������������
D�����ۺ���ά�ص����ڶ���C6H10O5�����ۺ϶Ȳ�ͬ���˴˲���ͬ���칹��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A������pH��11���ռ���Һ��pH��3��ϡ����������Ϻ���ʯ����Һ�ʺ�ɫ
B��T ��ʱ��pH=6�Ĵ�ˮ�У���10�C8 NA��OH����NA��ʾ�����ӵ�������
C����NaAlO2 ��FeCl3 ��Al2(SO4)3��ˮ��Һ�ֱ����������м��ȡ����ɲ����գ�
���ܵ�ԭ����
D����������ˮ�����c(H+)=1��10�C13mol/L����Һ��ܴ�������NH4+��Fe2+��NO3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���Ӫ�����ʵ�˵����ȷ����(����)
A����֬���⻯���ڻ�ԭ��Ӧ�������ڼӳɷ�Ӧ��������Ϊ������
B��1 mol���ǵ�ˮ�������1 mol�����Ǻ�1 mol����
C��������Һ��ϡ���Ṳ�Ⱥ���ˮ�ⷴӦ����ȴ�������������Һ��ˮԡ���Ⱥ���ֹ���������
D����������Һ�еμӱ��͵��������Һ�����ְ�ɫ�������ù��̽��������ʵı���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�ֶ��ĵĻ�ѧʽ��C8H14N2O5������ˮ�ⷴӦ��õ��������������һ�ְ�����X��X�Ļ�ѧʽΪ(����)
A��C4H7NO4 B��C6H7NO3
C��C5H9NO4 D��C5H11NO5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�蹸���ɷ�ֹ��ҵ��ˮ��������������Ṹ���������з�Ӧ·�߿ɵõ�E��R�����蹸��(���ַ�Ӧ������ȥ)��
(1)�蹸��E���Ʊ�
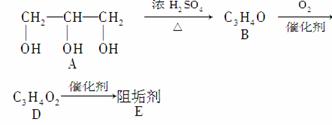
��A����������Ҫ��Ӫ������________ˮ���Ƶá�(����ࡱ������֬�������ʡ�)
��B�����Ƶ�Cu(OH)2����Һ��Ӧ����D���仯ѧ����ʽΪ
________________________________________________________________________
________________________________________________________________________��
��D���Ӿ۷�Ӧ����E��E�Ľṹ��ʽΪ
________________________________________________________________________
________________________________________________________________________��
(2)�蹸��R���Ʊ�
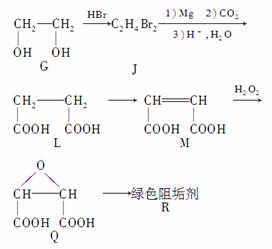
��G�D��JΪȡ����Ӧ��J�Ľṹ��ʽΪ
________________________________________________________________________��
��Jת��ΪL�Ĺ����У�L���������ӵ�̼ԭ����Դ��__________��
����L�Ʊ�M�ķ�Ӧ��������Ϊ��HOOCCH2CH2COOH��Br2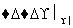
HOOCCH2CHBrCOOH��HBr��______________________
________________________________________________________________________��
________________________________________________________________________
_______________________________________(�û�ѧ����ʽ��ʾ)��
��1 mol Q��ͬ���칹��T(̼����֧��)������NaHCO3��Һ���ò���2 mol CO2��T�Ľṹ��ʽΪ__________________________________(ֻдһ��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵�����ʾ������ȷ����(��)
A.��Ӧ��������������������������ʱ,�÷�Ӧһ�����ܷ���
B.��ʯī�Ƚ��ʯ�ȶ���֪C(���ʯ,s) C(ʯī,s)����H<0
C(ʯī,s)����H<0
C.��֪:2SO2(g)+O2(g) 2SO3(g)����H=
2SO3(g)����H=
-98.3 kJ·mol-1����1 mol SO2��0.5 mol O2����һ�ܱ������г�ַ�Ӧ,�ų�49.15 kJ������
D.��101 kPa��25 ��ʱ,1 g H2��ȫȼ��������̬ˮ,�ų�120.9 kJ������,��������ȼ
��ʱ,1 g H2��ȫȼ��������̬ˮ,�ų�120.9 kJ������,��������ȼ ����Ϊ241.8 kJ·mol-1
����Ϊ241.8 kJ·mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л������У���ͬ��ԭ����PMR�ж�Ӧ��ͬ�ķ壨�źţ����ҷ��ǿ���������ͬ����ԭ�Ӹ��������ȡ����������������ֲ�ͬ��H�����е�H���Ǽ��е�H����PMR�������壬���������ǿ�ȱ�Ϊ6��2����д��3��1��ǰ��Ϊ�������Ӧ�ķ壬����Ϊ�Ǽ������Ӧ�ķ壩��ͬ�����״����ӵ�PMR�������壬��ǿ�ȱ�Ϊ3��1��
��1��������ӵ�PMR�� ���壻������( ��COOH)���ӵ�PMR�� ���塣
��COOH)���ӵ�PMR�� ���塣
��2��������PMR��ȷ��C2H6O�ṹʱ���ں˴Ź������ϸ����ķ������飬����һ��ֻ��һ���źŷ壬�����źŷ��Ӧ���ʵĽṹ��ʽΪ ����һ���������źŷ壬�������źŷ��ǿ�ȱ�Ϊ ��
��3����ѧʽΪC5H12�������ж��֣�������һ���ں˴Ź������ϵ��źŷ���4���������ʵ��ṹ��ʽΪ ������һ���ں˴Ź������ϵ��źŷ�ֻ��1��������������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ƕ���������ͬ���칹�壺
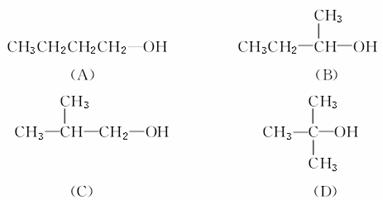
������Ҵ�����ȥ��Ӧ����������Ӧ�б仯�Ľṹ�ص㣬�ش���������(���¸���ֻ����д���)��
(1)����ȥ��Ӧ���ܹ��õ�������ȥ����Ľṹ��________________________________________________________________________��
(2)ֻ�õ�һ����ȥ����Ľṹ��________________________________________________________________________��
(3)�ܱ�����Ϊ����̼ͬԭ������ȩ�Ľṹ��________________________________________________________________________��
(4)���ܱ����ȵ�CuO�����Ĵ��Ľṹ��________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com