
��CH4����ԭNOx�������������������Ⱦ����֪CH4(g)��4NO2(g)===4NO(g)��CO2(g)��2H2O(g)����H����574 kJ·mol��1��CH4(g)��4NO(g)===2N2(g)��CO2(g)��2H2O(g)����H����1 160 kJ·mol��1�����ڱ�״����4.48 L CH4ǡ���ܽ�һ����NO2��ԭ��N2��H2O(g)�������������зų�������Ϊ(����)
A��114.8 kJ B��232 kJ
C��368.8 kJ D��173.4 kJ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ֱ���һ���Լ���ȥ���и������е�����(������Ϊ����)����д�����ӷ���ʽ��
(1)BaCl2��Һ(HCl)
�Լ���________�����ӷ���ʽ��__________________________ ______________________________________________��
(2)CO2(HCl)
�Լ���________�����ӷ���ʽ��__________________________ ______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ʒ������Ʒ�ڿ����в��ױ���ʴ��ԭ����(����)
A�������ܶȱ������ܶ�С
B�����Ľ�����Ա�����
C�����ڿ���������������Ӧ�γ�һ�����ܵ�����Ĥ
D�����ڳ����²�������������ѧ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijʵ��С����ͭ��[CuCO3��Cu(OH)2]�����ۺ�ϡ������ȡͭ��ʵ��������£�����ͭ�̵õ�һ�ֺ�ɫ���壬��������ϡ���ᷴӦ�Ƶõ�����ͨ���ɫ�����в����ȣ���ַ�Ӧ��õ�ͭ��
(1)����ʵ��������������Ļ�ѧ����ʽΪ��
______________________________________________________��
______________________________________________________��
______________________________________________________��
(2)ijͬѧ������ʵ����̸�Ϊ����ͭ����������ϡ�����У�Ȼ������������ۣ���ַ�Ӧ���ˣ�Ҳ���Եõ�ͭ�����������Ļ�ѧ����ʽΪ��
______________________________________________________��
______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ӦA��B����C(��H<0)���������У�
��A��B����X(��H>0)����X����C(��H<0)��
����ʾ��ͼ�У�����ȷ��ʾ�ܷ�Ӧ�����������仯����(����)
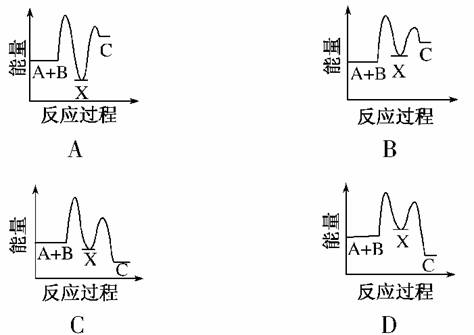
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��CaCO3(s)===CaO��CO2(g)����H��177.7 kJ
��C(s)��H2O(s)===CO(g)��H2(g)
��H����131.3 kJ/mol
��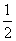 H2SO4(l)��NaOH(l)===
H2SO4(l)��NaOH(l)===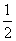 Na2SO4(l)��H2O(l)
Na2SO4(l)��H2O(l)
��H����57.3 kJ/mol
��C(s)��O2(g)===CO2(g)����H����393.5 kJ/mol
��CO(g)��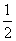 O2(g)===CO2(g)����H����283 kJ/mol
O2(g)===CO2(g)����H����283 kJ/mol
��HNO3(aq)��NaOH(aq)===NaNO3(aq)��H2O(l)
��H����57.3 kJ/mol
��2H2(g)��O2(g)===2H2O(l)����H����517.6 kJ/mol
(1)�����Ȼ�ѧ����ʽ�У�����ȷ����________������ȷ�����ɷֱ���_______________________________________________________________��
(2)����������Ϣ��д��Cת��ΪCO���Ȼ�ѧ����ʽ_____________��
(3)������Ӧ�У���ʾȼ���ȵ��Ȼ�ѧ����ʽ��________________����ʾ�к��ȵ��Ȼ�ѧ����ʽ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ҹ���ѧ���������ϵ�һ��Ϊһ����Ϊ����̪ݼ���ķ���(ֱ��Ϊ1.3����)�ָ��˴���,����̪ݼ�����ӽṹ�������������Ѫ���ؼ�ֲ�����ڵ�Ҷ���طdz����ơ����й��ڡ���̪ݼ�����ӵ�˵������ȷ����
A�����ȷ�ɢ��ˮ�����γɵķ�ɢϵ������Һ B������ֱ����Na+С
C�����ȷ�ɢ��ˮ���γɵķ�ɢϵ�ܲ��������ЧӦD������̪ݼ�����Ӳ�������ֽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵķ�����ȷ���ǣ� ��
A��SO2��CO2��CO��NO2��������������
B��Na2O��Na2O2��Fe2O3��CuO���Ǽ���������
C��HCl��HClO��HNO3��HClO4����ǿ��
D��NaOH��KOH��Ba(OH)2��Ca(OH)2����ǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ԫ�����ڱ���Ԫ�������ɣ������ƶ���ȷ����(����)
A��H3BO3�����Ա�H2CO3��ǿ
B��Mg(OH)2�ļ��Ա�Be(OH)2��ǿ
C��HCl��HBr��HI���� �ȶ���������ǿ
�ȶ���������ǿ
D����M����R2���ĺ�����Ӳ�ṹ��ͬ����ԭ��������R>M
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com