
����Ŀ����ͼ��ʾ��������֮���ת����ϵ������A��B��Ϊ��ɫ��ĩ��BΪ�ǽ������ʣ�CΪ��ɫ�����壬DΪ�������ʣ�E�Ǻ���ɫ���壬G�Ǿ���Ư���Ե����壬H��ˮ��Һ����ɫ��

��ش��������⣺
��1��A�Ļ�ѧʽ�� ��C�ĵ���ʽ�� ��Y�������� ��
��2����Ӧ1�Ļ�ѧ����ʽΪ ��
��3��19.2g��D��������һ��Ũ��X����Һ��Ӧ�������õ��������� LO2����״��������ϣ�ǡ���ܱ�ˮ��ȫ���ա�
���𰸡���1��CuO��![]() ��Ũ��������2��C��4HNO3��Ũ��
��Ũ��������2��C��4HNO3��Ũ��![]() CO2����4NO2����2H2O��
CO2����4NO2����2H2O��
��3��3.36��
��������
���������������֪������ȷ��A��CuO��B��C��C��CO2��D��Cu��E��NO2��F��H2O��G��SO2��H��CuSO4��I��NO��X��HNO3��Y��H2SO4����1��A�Ļ�ѧʽ��CuO��C�ĵ���ʽ��![]() ��Y��������Ũ�����2����Ӧ1��̼��Ũ�����ڼ���ʱ����������ԭ��Ӧ������CO2��NO2��H2O�����ݵ����غ㡢ԭ���غ㣬�ɵ÷�Ӧ�Ļ�ѧ����ʽΪC��4HNO3��Ũ��
��Y��������Ũ�����2����Ӧ1��̼��Ũ�����ڼ���ʱ����������ԭ��Ӧ������CO2��NO2��H2O�����ݵ����غ㡢ԭ���غ㣬�ɵ÷�Ӧ�Ļ�ѧ����ʽΪC��4HNO3��Ũ��![]() CO2����4NO2����2H2O����3��19.2g��Cu�����ʵ�����n��Cu��=19.2g��64g/mol=0.3mol����������������һ��Ũ��Ũ����X����Һ������Ӧ��Cu+ 4HNO3��Ũ��=Cu��NO3��2+NO2��+2H2O�������õ���������һ�������O2��ϣ�ǡ���ܱ�ˮ��ȫ���գ����ݵ���ת����Ŀ��ȣ���֪��Ӧ�����е���ת�Ƶ����ʵ�����n��e-��=0.3mol��2=0.6mol����Ӧ�����������ʵ�����n��O2��=0.6mol��4=0.15mol,���ڱ�״���������������V��O2��= 0.15mol��22.4L/mol=3.36L��
CO2����4NO2����2H2O����3��19.2g��Cu�����ʵ�����n��Cu��=19.2g��64g/mol=0.3mol����������������һ��Ũ��Ũ����X����Һ������Ӧ��Cu+ 4HNO3��Ũ��=Cu��NO3��2+NO2��+2H2O�������õ���������һ�������O2��ϣ�ǡ���ܱ�ˮ��ȫ���գ����ݵ���ת����Ŀ��ȣ���֪��Ӧ�����е���ת�Ƶ����ʵ�����n��e-��=0.3mol��2=0.6mol����Ӧ�����������ʵ�����n��O2��=0.6mol��4=0.15mol,���ڱ�״���������������V��O2��= 0.15mol��22.4L/mol=3.36L��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺

��1����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ������X�������۲쵽��������________________________________��Y�缫�ϵĵ缫��ӦʽΪ_______________��
��2����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ����X�缫�ĵ缫��Ӧʽ��______________________��Y�缫�IJ�����_______��
��3����Ҫ�õ�ⷽ��ʵ�����϶��������Һaѡ��_________��Һ����X�缫�IJ�����_______��Y�缫�ĵ缫��Ӧʽ��______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ij���̿�(��MnCO3��SiO2��FeCO3������Al2O3��)Ϊԭ��ͨ�����·����ɻ��̼���̴ֲ�Ʒ��
(��֪��Ksp(MnCO3)��2.2��10��11��Ksp[Mn(OH)2]��1.9��10��13��Ksp[Al(OH)3]��1.3��10��33��Ksp[Fe(OH)3]��4.0��10��38)
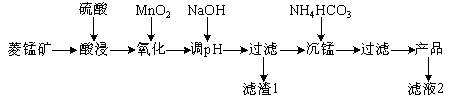
��1������1�У�����Ԫ�ص�������Ҫ�� (�ѧʽ����ͬ)����NaOH������Һ��pHԼΪ5�����pH�����ܵ�������1�� �������١�
��2����Һ2�У���1�������ӳ���H����� (�����ӷ���)��
��3��ȡ��������ǰ��Һa mL����ƿ�У���������AgNO3��Һ(������)������1.5%(NH4)2S2O8��Һ�����ȣ�Mn2��������ΪMnO����Ӧһ��ʱ��������5 min[��ȥ������(NH4)2S2O8]����ȴ�����¡�ѡ�����˵�ָʾ������b mol��L��1��(NH4)2Fe(SO4)2����Һ�ζ����յ㣬����(NH4)2Fe(SO4)2����ҺV mL��
��Mn2����(NH4)2S2O8��Ӧ�Ļ�ԭ����Ϊ (�ѧʽ)��
�ڡ�������ǰ��Һ��c(Mn2��)�� mol��L��1��
��4�������������䣬����������������Ԫ�ػ�������NH4HCO3��ʼŨ��(c0)����Ӧʱ��Ĺ�ϵ����ͼ��ʾ��

��NH4HCO3��ʼŨ��Խ����Ԫ�ػ�����Խ (����������������)������ԭ�� ��
������Һ��c(Mn2��)��1.0 mol��L��1����������1.8 mol��L��1 NH4HCO3��Һ���з�Ӧ������20��40 min��v(Mn2��)�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����˾��ƽ�������Ƹ�Ѫѹ��һ���ٴ�ҩ�����Ч�ɷ�M�Ľṹ��ʽ��ͼ��ʾ��

��1�����й���M��˵����ȷ����______(�����)��
a�����ڷ����廯���� b����FeCl3��Һ����ɫ
c����ʹ���Ը��������Һ��ɫ d��1molM��ȫˮ������2mol��
��2��������Ǻϳ�M���м��壬��һ�ֺϳ�·�����£�

����A������Ϊ______������I��B�IJ�������ƫ�ͣ���ԭ����__________��
������II��Ӧ�Ļ�ѧ����ʽΪ______________��
������III�ķ�Ӧ������________��
�������Ľṹ��ʽΪ__________________��
��C��ͬ���칹���ж��֣����б�������һ���������������_____�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��A���л���ѧ��ҵ�Ļ���ԭ�ϣ������������������һ�����ҵ�ʯ�ͻ�����չˮƽ��A����һ��ֲ���������ڼ���A�ɷ�����ͼ��ʾ��һϵ�л�ѧ��Ӧ�����Т٢ڢ�����ͬ�ַ�Ӧ���͡�������ͼ�ش��������⣺

��1��д��A��B��C��D�Ľṹ��ʽ��
A________��B________��C________��D________��
��2��д����������������Ӧ�Ļ�ѧ����ʽ����ע����Ӧ����
____________________����Ӧ����________��
____________________����Ӧ����________��
____________________����Ӧ����________��
��____________________����Ӧ����________��
��4����A��ȼ�շ�Ӧ����ʽ___________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼������������ѧ��ѧ��Ҫ�ķǽ���Ԫ�أ��ڹ�ũҵ�������й㷺��Ӧ����
��1�����ڷ������칬һ�����ij������Ż����ȼ����Һ̬ƫ�����£�CH3��2N��NH2����������Һ̬�����������������ڷ�Ӧ�����зų�����������ͬʱ������������Ⱦ�����塣��֪�����£�1 gȼ����ȫȼ���ͷų�������Ϊ42.5kJ����д���÷�Ӧ���Ȼ�ѧ����ʽ_________________��
��2��298 Kʱ����2L�����ܱ������з�����Ӧ��2NO2��g��![]() N2O4��g����H����a kJ��mol��1 ��a��0����N2O4�����ʵ���Ũ����ʱ��仯��ͼ1����ƽ��ʱ��N2O4��Ũ��ΪNO2��2�����ش��������⡣
N2O4��g����H����a kJ��mol��1 ��a��0����N2O4�����ʵ���Ũ����ʱ��仯��ͼ1����ƽ��ʱ��N2O4��Ũ��ΪNO2��2�����ش��������⡣

��298kʱ���÷�Ӧ��ƽ�ⳣ��Ϊ________��
�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ2��ʾ��
����˵����ȷ���ǣ� ��
a��A��C����ķ�Ӧ���ʣ�A��C
b��B��C����������ƽ����Է���������B��C
c��A��C�����������ɫ��A�Cdz
d����״̬B��״̬A�������ü��ȵķ���
������Ӧ��398K���У�ijʱ�̲��n��NO2��=0.6 mol ��n��N2O4��=1.2mol�����ʱV������ V������������>����<������=������
��3��NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺������100 mL 0.1 mol��L��1NH4HSO4��Һ�еμ�0.1 mol��L��1NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ3��ʾ���Է���ͼ��a��b��c��d��e����㣬
��ˮ�ĵ���̶�������__________������a����b����c����d������e������ͬ��
������Һ��c��OH-������ֵ��ӽ�NH3��H2O�ĵ��볣��K��ֵ���� ��
����c�㣬��Һ�и�����Ũ���ɴ�С������˳����_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.һ���¶��£�ij�ݻ�Ϊ2L���ܱ������ڣ�ijһ��Ӧ��M��N�����ʵ����淴Ӧʱ��仯��������ͼ����ͼ��ʾ��

��1���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2����ͼ����ʾ������ʱ���У� ����t1��t2��t3��ʱ�̴ﵽ��ѧ��Ӧ�ȡ�
II.һ���¶��½�6mol��A��6molB�����2L���ܱ������У��������·�Ӧ��3A��g��+B��g��![]() xC��g�� + 2D��g��������5���Ӻ�Ӧ�ﵽƽ�⣬���A��ת����Ϊ60����C��ƽ����Ӧ������0.36mol/��Lmin����
xC��g�� + 2D��g��������5���Ӻ�Ӧ�ﵽƽ�⣬���A��ת����Ϊ60����C��ƽ����Ӧ������0.36mol/��Lmin����
��1��ƽ��ʱD��Ũ��= mol/L��
��2��B��ƽ����Ӧ���ʦԣ�B��= mol/�� L.min����
��3��x= ��
��4����ʼʱ�����е�ѹǿ��ƽ��ʱ��ѹǿ֮��Ϊ ������Ϊ��������ȣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ұ���һ����;�㷺���л�ԭ�ϣ����Ʊ����ֻ�����Ʒ��
��һ���Ʊ�����ϩ��ԭ���練ӦI��ʾ����

��1�����ֻ�ѧ���ļ������±���ʾ��

���ݷ�ӦI�������仯������x= ____��
��2����ҵ�ϣ��ں�ѹ�豸�н��з�ӦIʱ�������ұ�������ͨ��һ������ˮ�������û�ѧƽ�����۽���ͨ��ˮ������ԭ��Ϊ____ ��
��3������ϵ�����ܱ仯�ĽǶȷ�������ӦI��____������¡����¡��������������Է����С�
�������Ʊ���-���һ�����ԭ���練ӦII��ʾ����

��4��T��ʱ����10 L�����ܱ������г���2mol�ұ�(g)��2 mol Cl2(g)������Ӧ��5 minʱ�ﵽƽ�⣬�ұ���Cl2����-���һ�����HCl�����ʵ���Ũ��(c)��ʱ��(t)�仯��������ͼl��ʾ��

��0��5 min�ڣ���HCl��ʾ�ĸ÷�Ӧ����v(HCl)=_____________��
��T��ʱ���÷�Ӧ��ƽ�ⳣ��K=_____________��
��6 minʱ���ı���������Ϊ_____________��
��10 minʱ�����������������䣬���������г���1moI�ұ���1 mol Cl2��1 mol ��-���һ�����l mol HCl��12 minʱ�ﵽ��ƽ�⡣��ͼ2�л���10-12 min��Cl2��HCl��Ũ�ȱ仯���ߣ������ϱ���Cl2��HC1����0��5 min��0��12 minʱ��Σ�Cl2��ת���ʷֱ��æ�1����2��ʾ�����l ��2(�>������<����=��)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ԭ���������������A��B��C��D��E��F����Ԫ�ء�����A�Ļ�̬ԭ����3����ͬ�ܼ������ܼ��еĵ�������ȣ�C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ�ӵ���ͬ��DΪ������������ԭ�Ӱ뾶��������Ԫ�أ�E��F��Cλ��ͬһ���壬F���ڵ�һ�������ڡ�
��1��Fԭ�ӻ�̬����Χ��������Ų�ʽΪ______________________________��
��2����A��B��C�γɵ�����CAB-��AC2��Ϊ�ȵ����壬��CAB-�ĽṹʽΪ_______________��
��3����Ԫ��A��E���γɵij����������У�Aԭ�ӹ�����ӻ�����Ϊ_______________��
��4����B��C��D����Ԫ���γɵĻ����ᄃ��ľ�����ͼ��ʾ����û�����Ļ�ѧʽΪ_________��

��5��PM2.5�����������ж����к����ʣ����������ι⻯ѧ������Ⱦ���⻯ѧ�����к���NOx��CH2=CHCHO��HCOOH��CH3COONO2(PAN)�ȶ�����Ⱦ�
������˵����ȷ����__________________��
A��N2OΪֱ���ͷ���
B��C��N��O�ĵ�һ��������������
C��CH2=CHһCHO������̼ԭ�Ӿ�����sp2�ӻ�
D����ͬѹǿ�£�HCOOH�е��CH3OCH3�ߣ�˵��ǰ���Ǽ��Է��ӣ������ǷǼ��Է���
��NO�ܱ�FeSO4��Һ�������������[Fe(NO)(H20)5]S04����������������ӵ�����Ϊ_____________�������ṩ�չ������__________________(��������)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com