
����Ŀ�������������ʵ�ת����ϵͼ���ش��й����⣺

��1�����������У�_________����ṹ��ʽ����ͬ���IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־�����������Ե�ζƷ�к���3%~5%��_________��������ζ����״Һ����_____________��
��2����ϩ�Ĺ�ҵ�Ʒ���_________��
a��ʯ���ѻ� b��ú���� c��ʯ���ѽ� d��ʯ�ͷ���
��3����ȩ�еĹ�����������______������ȩ��һ��̼ԭ�ӵ�ͬϵ��Ľṹ��ʽ��______��
��4����ҵ������ϩˮ�������Ҵ�����ϩ�ڼ��ȡ���ѹ�ʹ������ڵ������£���ˮ��Ӧ�����Ҵ����÷�Ӧ�Ļ�ѧ����ʽ��_______________________��
��5���������������Ļ�ѧ����ʽ��__________________________��
���𰸡�CH2=CH2 CH3COOH CH3COOCH2CH3 c ȩ�� HCHO CH2=CH2+H2O![]() CH3CH2OH CH3COOH+CH3CH2OH
CH3CH2OH CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O
��������
��1����ϩ��CH2=CH2���IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־�����������Ե�ζƷ��ʳ�ף����к���3%~5%�Ĵ��ᣨCH3COOH����һ���������ʾ�����ζ��������ζ����״Һ��������������CH3COOCH2CH3����
����CH2=CH2 ��CH3COOH��CH3COOCH2CH3
��2��a��ʯ���ѻ�����һ���������£�����Է��������ϴе�ϸߵ�������Ϊ��Է���������С���е�ϵ͵����Ĺ��̣��õ���С�������ʵ���Է���������Ȼ�ϴ�Ҫ�ٽ�һ���ѽ���ܻ����ϩ����a����
b��ú����ָú�ڸ������������¼��ȡ��ֽ⣬���ɽ�̿����뽹����ú���͡��ֱ���ú���Ȳ���Ĺ��̣�������Ϊ��ȡ��ϩ�ķ�������b����
c��ʯ���ѽ���һ�ָ���ȵ�ʯ���ѻ���ʯ���ѽ����ɵ��ѽ����dzɷָ��ӵĻ�����壬��Ҫ��Ʒ��ϩ����c��ȷ��
d��ʯ�ͷ����ǽ�ʯ���м��ֲ�ͬ�е�Ļ��������һ�ַ��������������仯���õ��������Ȼ�ǻ���������Ϊ��ȡ��ϩ�ķ�������d ����
��ѡc��
��3����ȩ�еĹ�����������ȩ��������ȩ��һ��̼ԭ�ӵ�ͬϵ���Ǽ�ȩ���ṹ��ʽ��HCHO��
��Ϊ��ȩ����HCHO��
��4����ҵ������ϩˮ�������Ҵ�����ϩ�ڼ��ȡ���ѹ�ʹ������ڵ������£���ˮ��Ӧ�����Ҵ����÷�Ӧ�Ļ�ѧ����ʽ��CH2=CH2+H2O![]() CH3CH2OH��
CH3CH2OH��
����CH2=CH2+H2O![]() CH3CH2OH��
CH3CH2OH��
��5���Ҵ���������Ũ������ȵ�����������������������ѧ����ʽ��CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
����CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����˾�����Ԥ���������������Ķ����ٻ��Ѫѹ��һ��ҩ���˾�����һ�ֺϳ�·�����£�

�ش��������⣺
(1)������Ľṹ��ʽΪ_______��E�к��������ŵ�������_______��
(2)D����E�ķ�Ӧ����Ϊ____________��
(3)C�Ľṹ��ʽΪ________________��
(4)A��FeCl3��Һ������ɫ��Ӧ��1 mol A��1 mol����������ड������з�Ӧ����1 mol B��1 mol H2O��1 mol CO2��B������ˮ�����ӳɷ�Ӧ���Ʋ�A����B�Ļ�ѧ����ʽΪ____��
(5)X��B��ͬ���칹�壬Xͬʱ�������������Ľṹ����____�֣����к˴Ź��������������Ľṹ��ʽΪ____��
�ٿ���̼��������Һ��Ӧ���ɶ�����̼
����FeCl3��Һ������ɫ��Ӧ
�۳������ⲻ��������
(6)д���Ա��״��ͱ�����Ϊԭ���Ʊ�![]() �ĺϳ�·��________(�����Լ���ѡ)��
�ĺϳ�·��________(�����Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ҹ������������Ĵ�����ӣ���������������˺ܴ����Ⱦ������β��װ��������ڴ�������������������õĹ�����ͼ��ʾ������˵����ȷ���ǣ� ��

A. ��Ӧ��NOΪ��������N2Ϊ��������
B. ����β������Ҫ��Ⱦ�ɷְ���CO��NO��N2
C. NO��O2�����ڴ���������ܷ�Ӧ
D. ��ת���ܻ�ѧ����ʽΪ2NO��O2��4CO ![]() 4CO2��N2
4CO2��N2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���������ܱ������г���H2��I2,������Ӧ H2(g)+I2(g)![]() 2HI(g)����H>0�ﵽƽ���t0ʱ�ı䷴Ӧ��ijһ����������������ʵ������䣩�������������ѹǿ��������˵����ȷ����
2HI(g)����H>0�ﵽƽ���t0ʱ�ı䷴Ӧ��ijһ����������������ʵ������䣩�������������ѹǿ��������˵����ȷ����
A.������������ɫ���ƽ����Է�����������
B.ƽ�ⲻ�ƶ�����������ܶȲ���
C.����ѹǿ����,����H2��I2(g),HIƽ��Ũ�ȶ�����
D.�ı�����������£�����ͼ��Ϊ��ͼ

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ����������β���ŷų���NOx��SO2�ȣ����γ���������Ҫ���ʣ����ۺ������ǵ�ǰ��Ҫ���о����⡣
��1����֪����COȼ���ȵ���H1=��283.0kJ��mol-l����N2(g)+O2(g) ![]() 2NO(g) ��H2=+180.5kJ��mol-1������β���е�NO(g)��CO(g)��һ���¶Ⱥʹ��������¿ɷ������·�Ӧ��2NO(g)+2CO(g)
2NO(g) ��H2=+180.5kJ��mol-1������β���е�NO(g)��CO(g)��һ���¶Ⱥʹ��������¿ɷ������·�Ӧ��2NO(g)+2CO(g) ![]() N2(g)+2CO2(g)�� ��H=___��
N2(g)+2CO2(g)�� ��H=___��
��2����0��20mol NO��0��10molCO����һ���ݻ��㶨Ϊ1L���ܱ������з���������Ӧ����Ӧ�����в������ʵ�Ũ�ȱ仯����ͼ��ʾ��
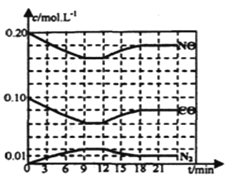
���÷�Ӧ��һ�δﵽƽ��ʱ��ƽ�ⳣ��Ϊ________��
����12minʱ�ı��������________��
���ڵ�24minʱ���������¶Ȳ��䣬���������г���CO��N2��0��060mol��ƽ�⽫________�ƶ�(����������������������������)��
(3)SNCR-SCR����������һ�����͵ij�ȥ�����е������������������һ����ð��������ء�
��SNCR���������У��ڴ�����������NH3����ԭ����ԭNO������Ҫ��ӦΪ��4NH3(g)+4NO(g)+O2(g)=4N2(g)+6H2O(g)����H<0����ϵ�¶�ֱ��Ӱ��SNCR����������Ч�ʣ���ͼ��ʾ������ϵ�¶�ԼΪ925��ʱ��SNCR����Ч����ߣ�����ܵ�ԭ����________��
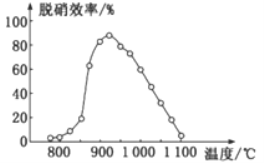
��SCR������������������[CO(NH2)2]����ԭ����ԭNO2�Ļ�ѧ����ʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Zn��һ��Ӧ�ù㷺�Ľ���������п��(��Ҫ�ɷ�ΪZnS��������SiO2������FeS��CdS��PbS���黯�������ʵ�)Ϊԭ���Ʊ�����Zn��ZnSO4��7H2O��������ͼ��ʾ��

����ؽ�������[c(Mn��)��0.1 mol��L��1]�γ��������������pH��Χ���£�
�������� | Fe3+ | Fe2+ | Zn2+ | Cd2+ |
��ʼ������pH | 1.5 | 6.3 | 6.2 | 7.4 |
������ȫ��pH | 2.8 | 8.3 | 8.2 | 9.4 |
��FeAsO4������ˮ��ZnSO4��7H2O������ˮ�������ھƾ���
�ش��������⣺
(1)����1����Ҫ�ɷֳ�SiO2���______�����պ����������Ի�����ɵij���Σ��Ϊ______��
(2)�������ӹ����м���ZnO��������___________��
(3)�Ƶõ�ZnSO4��7H2O��ϴ�ӣ�ϴ�Ӿ���ʱӦѡ�õ��Լ�Ϊ____________��
(4)��Һ�е�Cd2������п�۳�ȥ����ԭ���ӹ����з�Ӧ�����ӷ���ʽΪ___________������ʡȥ����ԭ���������裬ֱ���������������������г�ȥCd2����������________��
(5)�������õ�Cd�������������Ӽ��Զ��ε�أ���ع���ʱ������NiO(OH)ת��ΪNi(OH)2������ʱ��ص�������ӦʽΪ_____________������п��ĵ��Һ�ɷ���______�������ʹ�á�
(6)���Һ����Ԫ����AsO33�����ڣ�����������ʱ��������KMnO4��Һ��KMnO4����AsO33��������Ӧ����FeAsO4��д���÷�Ӧ�����ӷ���ʽΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�����Ȼ�ѧ����ʽ����CH4��ȼ����Ϊ(����)
![]() CH4(g)��O2(g)=
CH4(g)��O2(g)= ![]() CO2(g)��H2O(l)����H����445.15 kJ��mol��1
CO2(g)��H2O(l)����H����445.15 kJ��mol��1
CH4(g)��![]() O2(g)=CO(g)��2H2O(l)����H����607.3 kJ��mol��1
O2(g)=CO(g)��2H2O(l)����H����607.3 kJ��mol��1
CH4(g)��2O2(g)=CO2(g)��2H2O(l)����H����890.3 kJ��mol��1
CH4(g)��2O2(g)=CO2(g)��2H2O(g)����H����802.3 kJ��mol��1
A.445.15 kJ��mol��1B.890.3 kJ��mol��1
C.607.3 kJ��mol��1D.802.3 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�����ӷ���ʽ����д��ȷ����
A.����������Һ��ϡ���ᷴӦ��SO![]() + Ba2+ �� BaSO4 ��
+ Ba2+ �� BaSO4 ��
B.������ͨ���Ȼ�������Һ�У�Cl2��2Fe2+��2Fe3+��2Cl![]()
C.��FeCl3��Һʴ��ӡˢ��·���ϵ�Cu��2Fe3+��3Cu��2Fe��3Cu2+
D.������̼��������Һ��Ӧ��2H+��CO![]() ��H2O+ CO2��
��H2O+ CO2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ȩ��һ��ʳƷ���Ӽ�����������ľ����ԭ�Ϻϳɣ��ϳ�·�����£�
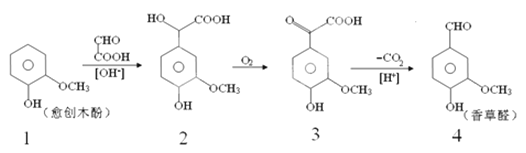
����˵����ȷ���ǣ�������
A. ��Ӧ1��2���ڼӳɷ�Ӧ�������ɵĻ�����2����һ������̼ԭ��
B. ������2��һ�������¿ɷ�����ȥ��Ӧ
C. �����Ƶõ����ȩ���Ƿ���л�����3�������Ȼ�����Һ
D. �����ʵ������ֻ�����ֱ�������NaOH��Ӧ������NaOH���ʵ���֮��Ϊ1��3��2��4
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com