
| A�� | ������Һ�е�KW��ͬ����ˮ�������c��H+������=��=��=�� | |
| B�� | ȡ�����ͬ����Һ�١��ڡ��۷ֱ����������۷�Ӧ������H2������������ | |
| C�� | ���������������Һ�ֱ�ϡ��100����������Һ��pH���ۣ��ܣ��ڣ��� | |
| D�� | �����£����ں͢۵������ϣ�c��CH3COO-��-c��Na+��=c��H+��-c��OH-�� |
���� A���¶Ȳ��䣬ˮ�����ӻ��������䣬������Һ��PHֵ����ˮ�����������Ũ�ȣ�
B���ȸ�����Һ��PHֵ�ж���Һ�����ʵ���Ũ�ȣ�����ȡ��Һ�����ͬʱ���ж����ʵ����ʵ�����Դ�С�Ӷ��ж���������Ķ��٣�
C�����ݵ���ʵ�ǿ������Һϡ��ʱ����Ũ�ȵı仯�ж���Һ��PHֵ��С��
D�����ں͢۵������ϣ������������Һ������Ϊ����ʹ����ƣ���ϵ���غ������
��� �⣺A����ͬ�����£�ˮ�����ӻ������Ƕ�ֵ�������ỹ�Ǽ����ˮ�ĵ��룬��pH=2��HCl��Һ����pH=2��CH3COOH��Һ����pH=12��NaOH��Һ����pH=12�İ�ˮ���١��ڵ�������Ũ����ͬ���ۡ��ܵ����������ӵ�Ũ����ͬ��������������Һ����ˮ�����c��H+������=��=��=�ܣ���A��ȷ��
B�����������ᣬ�Ȼ��������������ǿ����ʣ��١��ڡ���������Һ�����ʵ���Ũ�ȹ�ϵΪ���ڣ���=�ۣ����Ե�����Ģ١��ڡ�����Һ�ֱ������۷�Ӧ��2mol������������6mol������ų�������ȣ���������H2�����������٣���B��ȷ��
C�����������ᣬ��ˮϡ�ͺ��ܴٽ�����ĵ��룬���Ԣ١���ϡ�ͺ���Һ��pHֵ7���ڣ��٣���ˮ�������ˮϡ�ͺ��ܴٽ���ˮ�ĵ��룬���Ԣۡ���ϡ�ͺ���Һ��PHֵ�ܣ��ۣ�7��������������������Һ�зֱ����100mLˮ����Һ��pH���ܣ��ۣ��ڣ��٣���C����
D�����ں͢۵������ϣ������������Һ������Ϊ����ʹ����ƣ���Һ�е���غ�Ϊc��CH3COO-��+c��OH-��=c��H+��+c��Na+������c��CH3COO-��-c��Na+��=c��H+��-c��OH-������D��ȷ��
��ѡC��
���� ���⿼����������ʵĵ��롢��Һϡ�ͺ�PHֵ��Դ�С�ıȽϵ�֪ʶ�㣬ע��������ˮ��Һ���Ǽ���Һ������ˮ�ĵ��룬�����ˮ���ܴٽ�ˮ�ĵ��룬��Ŀ�ѶȲ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������и߶���9�µ��л�ѧ�Ծ��������棩 ���ͣ�ѡ����
���淴Ӧ��2NO2(g�� 2NO(g����O2(g����������̶����ܱ������У��ﵽƽ��״̬�ı�־�ǣ� ��
2NO(g����O2(g����������̶����ܱ������У��ﵽƽ��״̬�ı�־�ǣ� ��
�ٵ�λʱ��������n mol O2 ��ͬʱ����2n mol NO2
��ͬʱ����2n mol NO2
�ڵ�λʱ��������n mol O2��ͬʱ����2n mol NO
����NO 2��NO��O2��ʾ�ķ�Ӧ���ʵı�Ϊ2��2��1��״̬
2��NO��O2��ʾ�ķ�Ӧ���ʵı�Ϊ2��2��1��״̬
�ܻ���������ɫ���ٸı��״̬
�ݻ��������ܶȲ��ٸı��״̬
��������ѹǿ���ٸı��״̬
��������ƽ����Է����������ٸı��״̬
A���٢ܢޢ� B���ڢۢݢ� C. �٢ۢܢ� D��ȫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �٢� | D�� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| �� | �� | �� | �� | |
| pH | 12 | 12 | 2 | 2 |
| ��Һ | ��ˮ | ����������Һ | ���� | ���� |
| A�� | �ڢ١�������Һ�зֱ�����Ȼ�茶��壬����Һ��pH������ | |
| B�� | ��������Ģٺ͢�����Һ�ֱ��ˮϡ��100����������Һ��pH���٣��� | |
| C�� | �Ѣ١�������Һ�������Ϻ�������Һ�У�[Cl-]��[NH4+]��[OH-]��[H+] | |
| D�� | ����Һ�ں���Һ�۵������ϣ���Ϻ�������Һ��pH=7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 CH3COOHΪ���������ᣬ�ڹ�ҵ�������������й㷺��Ӧ�ã�
CH3COOHΪ���������ᣬ�ڹ�ҵ�������������й㷺��Ӧ�ã��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
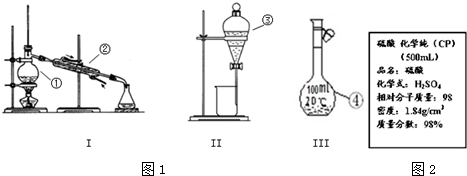
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
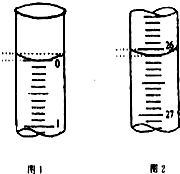 ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ�������ָʾ��������д���пհף�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ�������ָʾ��������д���пհף�| �ζ����� | ��������������Һ�����/mL | 0.1000mol•L-1��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com