
��14�֣���ͼ��X����֧���ġ����й���ζ�ĺϳ����ϣ������ڵ�����ֹ������㾫����֪D�ڱ�״���µ��ܶ�Ϊ1.25 g/L�������������������һ������ʯ�ͻ�����չˮƽ��E�������г�����һ���л�������ʼ�ת����ϵ���£�
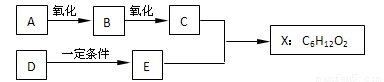
��ش��������⡣
��1��A���ӵ������� ��
��2��B������������������ ��
��3��C+E��X�Ļ�ѧ��Ӧ������ ��Ӧ��
��4��д������������A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ������A����
�� ��
��5��X������������Һ��Ӧ�Ļ�ѧ����ʽ ��
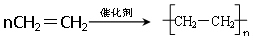
��DΪԭ������һ�ֳ������ϵĻ�ѧ����ʽ�� ��
��1������ ��2��ȩ�� ��3������
��4��CH3CH2CHOHCH3��(CH3)2CHCH2OH��(CH3)3COH
��5��CH3CH2
CH2 COOC2H5 + NaOH CH3CH2 CH2 COONa + C2H5OH
CH3CH2 CH2 COONa + C2H5OH
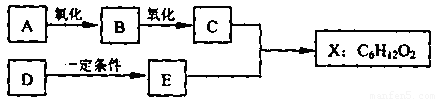
��������X���й���ζ�ĺϳ����ϣ�˵��X����������C�����ᣬE�Ǵ���D�ڱ�״���µ��ܶ�Ϊ1.25 g/L�������������������һ������ʯ�ͻ�����չˮƽ������D����ϩ����ϩ�ۼ��õ�����ϩ���ϡ���ϩ��ˮ�ӳɼ��õ��Ҵ���E���Ҵ�������C�Ƕ��ᡣ����ΪX����֧���ģ�����X�Ľṹ��ʽΪCH3CH2 CH2 COOC2H5����C��CH3CH2CH2COOH��B�Ľṹ��ʽ��CH3CH2CH2CHO��A�Ľṹ��ʽ��CH3CH2CH2CH2OH��������4��ͬ���칹�壬�������ֱַ���CH3CH2CHOHCH3��(CH3)2CHCH2OH��(CH3)3COH��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱�����������߶���ѧ����ĩ���Ի�ѧ���� ���ͣ������
(14��) ��ͼ��X����֧���ġ����й���ζ�ĺϳ����ϣ������ڵ�����ֹ������㾫����֪D�ڱ�״���µ��ܶ�Ϊ1.25 g/L�������������������һ������ʯ�ͻ�����չˮƽ��E�������г�����һ���л�������ʼ�ת����ϵ���£�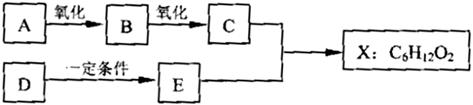
��ش��������⡣
��1��A�������� ��
��2��B�������������� ��
��3��C+E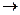 X�Ļ�ѧ��Ӧ������ ��Ӧ��
X�Ļ�ѧ��Ӧ������ ��Ӧ��
��4��д������������A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ(����A)��
�� ��
��5��X������������Һ��Ӧ�Ļ�ѧ����ʽ��
��
��6����DΪԭ������һ�ֳ������ϵĻ�ѧ����ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ�߶��µ������¿���ѧ�Ծ��������棩 ���ͣ������
��ͼ��X����֧���ġ����й���ζ�ĺϳ����ϣ������ڵ�����ֹ������㾫����֪
D�ڱ�״���µ��ܶ�Ϊ1.25 g/L�������������������һ������ʯ�ͻ�����չˮƽ��E������
�г�����һ���л�������ʼ�ת����ϵ���£�
��ش��������⡣
(1)A�������� ��
(2)B�������������� ��
(3)C+E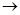 X�Ļ�ѧ��Ӧ������ ��Ӧ��
X�Ļ�ѧ��Ӧ������ ��Ӧ��
(4)д������������A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ (����A)�� ��
(5)X������������Һ��Ӧ�Ļ�ѧ����ʽ�� ��
(6)��DΪԭ������һ�ֳ������ϵĻ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱�����������߶���ѧ����ĩ���Ի�ѧ���� ���ͣ������
(14��) ��ͼ��X����֧���ġ����й���ζ�ĺϳ����ϣ������ڵ�����ֹ������㾫����֪D�ڱ�״���µ��ܶ�Ϊ1.25 g/L�������������������һ������ʯ�ͻ�����չˮƽ��E�������г�����һ���л�������ʼ�ת����ϵ���£�
��ش��������⡣
��1��A�������� ��
��2��B�������������� ��
��3��C+E X�Ļ�ѧ��Ӧ������
��Ӧ��
X�Ļ�ѧ��Ӧ������
��Ӧ��
��4��д������������A������ͬ�����ŵ�A��ͬ���칹��Ľṹ��ʽ (����A)��
�� ��
��5��X������������Һ��Ӧ�Ļ�ѧ����ʽ��
��
��6����DΪԭ������һ�ֳ������ϵĻ�ѧ����ʽ��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com