���𰸡�
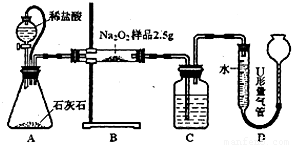
��������1�����������ܺͶ�����̼��Ӧ������������Ӧ��
��2����Ƥ�ܿ��Թ�ͨ��ƿ�ͷ�Һ©���еĴ���ѹǿ�������Ա��������ſ�����
��3�������������������ʵ��ֵ�ߣ���������ƵĴ���װ�þͻ�ƫ�ߣ�
��4����������ƿ��ȡҺ�Ļ���ʵ������жϣ�ȡҺ�õ�����Һ�ܣ�
��5�����ݱ������ı�Һ������ݣ��������Һ��ƽ����������ù�ϵʽ��Na
2O
2��2HCl��Na
2O��2HCl������������ơ��������ܵ����ʵ����������������ݣ���ʽ����������ơ��������Ƹ��Ե����ʵ��������������������Ƶ�����������
����⣺��1��C�����������ն�����̼����֤��ˮ��������õ��������������
�ʴ�Ϊ��NaOH��Һ��
��2��A����Ƥ�ܿ��Թ�ͨ��ƿ�ͷ�Һ©�������ѹ���ã�ʹ���������£����ҿ��Ա��������ſ���ʹ�������ƫ��
�ʴ�Ϊ�����ѹ���ã�ʹ���������£����������ſ���ʹ�������ƫ��
��3��a��װ��A��B�еĿ����ᵼ�²�õ��������ƫ�ߣ��Բⶨ�������Ӱ�죬��a��ȷ��
b���ڵ���U���������е�Һ����ƽ�Ĺ����У��ų���װ��C�еĿ����Բⶨ���������Ӱ�죬��b����
c������ʱU���������е�Һ��Ҫ������ƽ������ҵͣ�����Na
2O
2�Ĵ��Ȼ�ƫ��c��ȷ��
d������ʱU���������е�Һ������Ҹ�ʱ������Na
2O
2�Ĵ��Ȼ�ƫС����d����
��ѡac��
��4��ͼ�����õ���������Һ�ܣ�����Һ�ܷ��������Һ�������У�ʹ���ڼ�˿��������ڱڣ���������б����Һ���ִ�ֱ���ſ�ʳָ��ʹ��Һ�������ڱ���Ȼ���£�����Һ������Һ�������ٵȴ�15�룬ȡ����Һ�ܣ����ڹܿڵ�����Һ�岻Ҫ������
�ʴ��ǣ�D����Һ�ܣ�
��5�����εζ����ı�Һ���������Ч�ģ��������ĵı�Һ�����ƽ������ǣ�
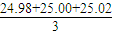
mL=25mL���������ơ������ƺ����ᷴӦ�Ĺ�ϵʽ�ǣ�Na
2O
2��2HCl��Na
2O��2HCl���������ƺ��������ܵ����ʵ����ǣ�n��Na
2O
2��=

n��HCl��=0.5×0.025L×0.1000mol?L
-1=0.00125mol��1000.00mL��Һ��Һ�к��е������ƺ��������ܵ����ʵ����ǣ�0.00125×
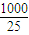
=0.05mol��
���������xmol�������������ʵ����ǣ�0.05-x��mol��
����������ϵ�ɵã�78x+62×��0.05-x��=3.500��
���x=0.025mol���������Ƶ����������ǣ�

×100%=55.7%��
�ʴ��ǣ�55.7%��
���������⿼��ѧ��ʵ�������Լ��������Ƶ����ʣ�Ҫ��ѧ�����з����ͽ��������������ѶȽϴ�


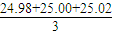 mL=25mL���������ơ������ƺ����ᷴӦ�Ĺ�ϵʽ�ǣ�Na2O2��2HCl��Na2O��2HCl���������ƺ��������ܵ����ʵ����ǣ�n��Na2O2��=
mL=25mL���������ơ������ƺ����ᷴӦ�Ĺ�ϵʽ�ǣ�Na2O2��2HCl��Na2O��2HCl���������ƺ��������ܵ����ʵ����ǣ�n��Na2O2��= n��HCl��=0.5×0.025L×0.1000mol?L-1=0.00125mol��1000.00mL��Һ��Һ�к��е������ƺ��������ܵ����ʵ����ǣ�0.00125×
n��HCl��=0.5×0.025L×0.1000mol?L-1=0.00125mol��1000.00mL��Һ��Һ�к��е������ƺ��������ܵ����ʵ����ǣ�0.00125×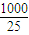 =0.05mol��
=0.05mol�� ×100%=55.7%��
×100%=55.7%��


 ���أ�
���أ� ������ɰ��Na2B4O7���ڸ��¸�ѹ�·�Ӧ���Ի�������
������ɰ��Na2B4O7���ڸ��¸�ѹ�·�Ӧ���Ի�������