
 ��1��ij�л��ﺬ̼85.7%����14.3%����80g����5%����ˮ��ͨ����л����ˮǡ����ȫ��ɫ����ʱҺ������81.4g��
��1��ij�л��ﺬ̼85.7%����14.3%����80g����5%����ˮ��ͨ����л����ˮǡ����ȫ��ɫ����ʱҺ������81.4g��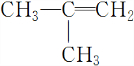 ����ѡ����һ��д�������ɸ߾���Ļ�ѧ����ʽ
����ѡ����һ��д�������ɸ߾���Ļ�ѧ����ʽ ����
����
 +3HNO3
+3HNO3
 +3H2O��
+3H2O�� ���� ��1��ij�л��ﺬ̼85.7%����14.3%��C��H��������֮��Ϊ100%�����л���ֻ��C��H����Ԫ�أ��������C��Hԭ����Ŀ֮��Ϊ$\frac{85.7%}{12}$��$\frac{14.3}{1}$=1��2���л���ʵ��ʽΪCH2����ˮ��ͨ����л����ˮǡ����ȫ��ɫ����������ϩ��������Br2�����ʵ���֮��1��1��Ӧ����n��ϩ����=n��Br2��=$\frac{80g��5%}{160g/mol}$=0.025mol��Һ������Ϊ81.4g-80g=1.4g��Ϊ�л������������������Է�������Ϊ$\frac{1.4g}{0.25mol}$=56�����л������ʽΪC4H8��
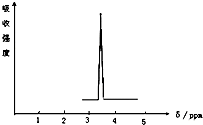
��2���˴Ź��������з�ֵ�������л�������ԭ�ӵ����������˴Ź���������ֻ����һ�ַ壬˵���÷�����ֻ��1��Hԭ�ӣ�Ȼ����ݷ���ʽд����Ӧ�Ľṹ��
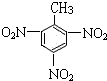
��3��ij������Է�������Ϊ92������������Cԭ����ĿΪ$\frac{92}{12}$=7��8����������ʽΪC7H8����������ʹ������Ȼ�̼��Һ��ɫ����ʹ���Ը��������ɫ�������Ϊ ��
��
��� �⣺��1����ij�л��ﺬ̼85.7%����14.3%��C��H��������֮��Ϊ100%�����л���ֻ��C��H����Ԫ�أ��������C��Hԭ����Ŀ֮��Ϊ$\frac{85.7%}{12}$��$\frac{14.3}{1}$=1��2���л���ʵ��ʽΪCH2����ˮ��ͨ����л����ˮǡ����ȫ��ɫ����������ϩ��������Br2�����ʵ���֮��1��1��Ӧ����n��ϩ����=n��Br2��=$\frac{80g��5%}{160g/mol}$=0.025mol��Һ������Ϊ81.4g-80g=1.4g��Ϊ�л������������������Է�������Ϊ$\frac{1.4g}{0.25mol}$=56�����л������ʽΪC4H8��
�ʴ�Ϊ��C4H8��
�ھ��ⶨ���л��������������-CH3�����Ľṹ��ʽΪ��CH3CH=CHCH3��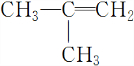 ��CH3CH=CHCH3�Ӿ۷�Ӧ�ķ���ʽΪ��
��CH3CH=CHCH3�Ӿ۷�Ӧ�ķ���ʽΪ�� ��
��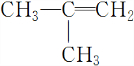 �����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ��
�����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ�� ��
��
�ʴ�Ϊ��CH3CH=CHCH3��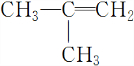 ��
�� ����
���� ����
����
��2���˴Ź���������ֻ����һ�ַ壬˵���÷�����ֻ��1��Hԭ�ӣ���A�Ľṹ��ʽΪCH2BrCH2Br��B�Ľṹ��ʽΪCH3CHBr2��������Hԭ�ӣ����Ժ˴Ź�����������2���壬
�ʴ�Ϊ��CH2BrCH2Br��2��
��3��ij������Է�������Ϊ92������������Cԭ����ĿΪ��$\frac{92}{12}$=7��8����������ʽΪC7H8����������ʹ������Ȼ�̼��Һ��ɫ����ʹ���Ը��������ɫ�������Ϊ ��
�� ��Ũ���ᷢ��ȡ����Ӧ����
��Ũ���ᷢ��ȡ����Ӧ���� ����Ӧ����ʽΪ��
����Ӧ����ʽΪ�� +3HNO3
+3HNO3
 +3H2O��
+3H2O��
�ʴ�Ϊ�� +3HNO3
+3HNO3
 +3H2O��
+3H2O��
���� ���⿼���л���ṹ�����ʣ���Ŀ�Ѷ��еȣ���ȷ�����л���ṹ������Ϊ���ؼ�����2������ȷ�˴Ź������ĺ���Ϊ���ؼ�������������ѧ���ķ������������Ӧ��������


 â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaHSO3��Һ�д��ڣ�c��Na+����c��HSO3-����c��OH-����c��H+����c��SO32-�� | |
| B�� | ������Һ������ʱ��c��Na+��=2c��SO32-��+c��HSO3-�� | |
| C�� | �������ʵ�����Na2SO3��NaHSO3����Һ�У���������Ŀ��� | |
| D�� | Na2SO3��NaHSO3��������ˮ�ĵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1 mol Cl2����ˮ�Ĺ�������1NA������ת�� | |
| B�� | ��״���£�22.4L NO2�����к���NA��O2���� | |
| C�� | ���³�ѹ�£�15g HCHO����2NA�Թ��õ��Ӷ� | |
| D�� | 1L 0.1mol•L-1�Ĵ�����Һ����NA��CH3COO- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �٢ڢ� | C�� | �ۢܢ� | D�� | �ڢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣�
Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ʵ��� | B�� | ��� | C�� | ���� | D�� | ��ԭ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʳƷ���г����й轺�����ۣ�������������� | |
| B�� | ������ˮʱ�����˻�ѧ�������仯������ˮ���ã���û��ɱ�������������� | |
| C�� | ����ʵʩ����ȼ�����������������ܼ�������������ŷ� | |
| D�� | ij���ͺ����������Ҫ�ɷ�����̼���衢�մɺ�̼��ά���϶��ɣ�����һ���������ǽ������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com