
ʯ�ͻ���ר���ɶ���Ժʿ��2007��ȹ�����߿�ѧ������������ʯ�ͻ��������������µ������ߺ���ɫ��ѧ�Ŀ����ߣ������ƵĶ���ʯ�����ƴ�������ؽ������ҹ�ʯ�ͻ�����Ʒ�ijɱ���
(1)ʹ�ô�������ʯ���ѻ���õ���Ҫ��Ʒ��________��ʯ���ѽ����ҪĿ����________________________________________________________________________��
(2)����ɫ��ѧ���ա�������״̬�Ƿ�Ӧ����ԭ��������Ϊ100%����ҵ�ϣ�ͨ����ɫ��ѧ������CO��CH3OH��һ�ֲ�����֬�������ϳ�CH2===C(CH3)COOCH3���ò�����֬�������ķ���ʽΪ________��
(3)������Ա��ʵ�����а����������ڴ���Ӧ����װ�ز�ͬ�Ĵ�����̽����ͬ������ʯ���ѽⷴӦ�Ĵ����ܡ�
 ��
�� ��
�� ��
��
�ٸ�̽��ʵ����������Ʒdz���Ҫ�����װ����ѡ��ļ���Լ�������ע���������____________��
�ڴӰ�ȫ�ĽǶȿ��ǣ���ʵ��β�������ķ�����________��
 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Լ��У�����ʹFe2��ת��ΪFe3������(����)
����������NaCl��Һ����KMnO4��Һ����ϡ���ᡡ�����ᡡ��NaNO3��Һ
A���٢ڢ� B���٢ۢ� C���ڢܢ� D���ڢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij���ķ���ʽΪC10H14��������ʹ��ˮ��ɫ������ʹ����KMnO4��Һ��ɫ�����ӽṹ��ֻ����һ���������������������(����)
A��2�� B��3��
C��4�� D��5��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���������(����)
A�������Ǻ���C5��C11������������ͨ��ʯ�͵ķ���õ�����
B����C18�������������;������ѻ����Եõ�����
C��ú�����л����������ɵĻ����
D��ú���б��ͼױ��������ȸ�������ķ��������Ƿ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪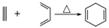 �����Ҫ�ϳ�
�����Ҫ�ϳ�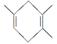 �����õ���ʼԭ�Ͽ�����(����)
�����õ���ʼԭ�Ͽ�����(����)
A��2��?1,3����ϩ��1��Ȳ
B��1,3���ϩ��2��Ȳ
C��2,3����?1,3���ϩ����Ȳ
D��2,3����?1,3����ϩ�ͱ�Ȳ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)____S��____KOH===____K2S��____K2SO3��____H2O
(2)____P4��____KOH��____H2O===____K3PO4��____PH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ȡx gͭþ�Ͻ���ȫ����Ũ�����У���Ӧ���������ᱻ��ԭֻ����8 960 mL��NO2�����672 mL��N2O4����(�������㵽��״̬)���ڷ�Ӧ�����Һ�м�������������������Һ�����ɳ�������Ϊ17.02 g����x����(����)
A��8.64 B��9.20
C��9.00 D��9.44
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ú���Al2O3��SiO2������FeO·xFe2O3�������Ʊ�Al2(SO4)3·18H2O��������������(���ֲ�����������)��
��.�������м������ϡH2SO4�����ˣ�
��.����Һ�м������KMnO4��Һ��������Һ��pHԼΪ3��
��.���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ��
��.����MnSO4���Ϻ�ɫ��ʧ�����ˣ�
��.Ũ�����ᾧ�����룬�õ���Ʒ��
(1)H2SO4�ܽ�Al2O3�����ӷ���ʽ��________________________________________________________________________
________________________________________________________________________��
(2)��MnO ����Fe2�������ӷ���ʽ����������
����Fe2�������ӷ���ʽ����������
 MnO
MnO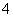 ��
�� Fe2����
Fe2���� ________===
________===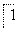 Mn2����
Mn2���� Fe3����
Fe3���� ________��
________��
(3)��֪��
�����������������pH
| Al(OH)3 | Fe(OH)2 | Fe(OH)3 | |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
ע���������ӵ���ʼŨ��Ϊ0.1 mol·L��1
���ݱ������ݽ��Ͳ�����Ŀ��________________________________________________________________________��
(4)��֪��һ�������£�MnO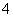 ����Mn2����Ӧ����MnO2��
����Mn2����Ӧ����MnO2��
�����ij����м���ŨHCl�����ȣ���˵�������д���MnO2��������________________________________________________________________________��
�ڢ��м���MnSO4��Ŀ����________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(����)
A�����Ǿ����˷��ӵĽṹ
B�����ۼ��ļ���Խ���ۼ�Խ�ι̣����иü��ķ���Խ�ȶ�
C��CH4��CCl4�м�����ȣ����Dz�ͬ
D��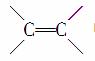 �еļ�����C��C�еļ��ܵ�����
�еļ�����C��C�еļ��ܵ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com