


 ӢI
”¢I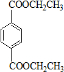 £®
£®
 £®
£®·ÖĪö ÓÉPETGµÄ½į¹¹¼ņŹ½æÉÖŖ¶ŌÓ¦µÄµ„ĢåÓŠHOCH2CH2OH”¢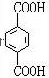 ”¢
”¢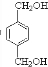 £¬ŌņDÓ¦ĪŖHOCH2CH2OH£¬FĪŖ
£¬ŌņDÓ¦ĪŖHOCH2CH2OH£¬FĪŖ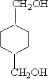 £¬HĪŖ
£¬HĪŖ £¬EĪŖ
£¬EĪŖ £¬GĪŖ
£¬GĪŖ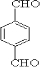 £¬BĪŖ
£¬BĪŖ £¬AĪŖCH2=CH2£¬CĪŖCH2BrCH2Br£¬IĪŖ
£¬AĪŖCH2=CH2£¬CĪŖCH2BrCH2Br£¬IĪŖ £¬½įŗĻÓŠ»śĪļµÄ½į¹¹ŗĶŠŌÖŹŅŌ¼°ĢāÄæŅŖĒóæɽā“šøĆĢā£®
£¬½įŗĻÓŠ»śĪļµÄ½į¹¹ŗĶŠŌÖŹŅŌ¼°ĢāÄæŅŖĒóæɽā“šøĆĢā£®
½ā“š ½ā£ŗÓÉPETGµÄ½į¹¹¼ņŹ½æÉÖŖ¶ŌÓ¦µÄµ„ĢåÓŠHOCH2CH2OH”¢ ”¢
”¢ £¬ŌņDÓ¦ĪŖHOCH2CH2OH£¬FĪŖ
£¬ŌņDÓ¦ĪŖHOCH2CH2OH£¬FĪŖ £¬HĪŖ
£¬HĪŖ £¬EĪŖ
£¬EĪŖ £¬GĪŖ
£¬GĪŖ £¬BĪŖ
£¬BĪŖ £¬AĪŖCH2=CH2£¬CĪŖCH2BrCH2Br£¬IĪŖ
£¬AĪŖCH2=CH2£¬CĪŖCH2BrCH2Br£¬IĪŖ £¬
£¬
£Ø1£©ÓÉŅŌÉĻ·ÖĪö½įŗĻĢāøų×Ŗ»Æ¹ŲĻµæÉÖŖ¢Ś¢Ū¢ßŹōÓŚČ”“ś·“Ó¦£¬¢Ł¢ÜĪŖ¼Ó³É·“Ó¦£¬ĘäĖūĪŖŃõ»Æ·“Ó¦£¬
¹Ź“š°øĪŖ£ŗ¢Ś¢Ū¢ß£»
£Ø2£©ÓÉŅŌÉĻ·ÖĪöæÉÖŖBĪŖ £¬IĪŖ
£¬IĪŖ £¬
£¬
¹Ź“š°øĪŖ£ŗ £»
£» £»
£»
£Ø3£©·“Ó¦¢ÜµÄ·“Ó¦ĪŖ £¬·“Ó¦¢ŻµÄ·“Ó¦ĪŖ
£¬·“Ó¦¢ŻµÄ·“Ó¦ĪŖ £¬
£¬
¹Ź“š°øĪŖ£ŗ £»
£» £»
£»
£Ø4£©ŗĻ³ÉŹ±Ó¦æŲÖʵĵ„ĢåµÄĪļÖŹµÄĮæn £ØD£©£ŗn £ØF£©£ŗn £ØH£©=m£ŗn£ŗ£Øm+n£©£¬
¹Ź“š°øĪŖ£ŗm£ŗn£ŗ£Øm+n£©£®
µćĘĄ ±¾Ģāæ¼²éÓŠ»śĪļµÄĶʶĻ£¬²ąÖŲӌѧɜ·ÖĪöÄÜĮ¦ŗĶĶʶĻÄÜĮ¦µÄ漲飬øĆĢāŠÅĻ¢Įæ“ó£¬×¢Ņāøł¾Żøß¾ŪĪļµÄ½į¹¹¼ņŹ½ÅŠ¶Ļµ„ĢåĪŖ½ā“šøĆĢāµÄĶ»ĘĘæŚ£¬“šĢāŹ±×¢Ņā°ŃĪÕĢāøųŠÅĻ¢£¬ĪŖ½ā“šøĆĢāµÄ¹Ų¼ü£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ³£ĪĀ³£Ń¹ĻĀ£¬1.6gCH4ŗ¬ÓŠµÄµē×ÓŹżÄæĪŖNA | |
| B£® | ±ź×¼×“æöĻĀ£¬2.2 LCCl4ŗ¬ÓŠµÄĢ¼Ō×ÓŹżÄæĪŖ0.1NA | |
| C£® | 101kPa”¢0”ꏱ£¬22.4LH2ÖŠĒāŌ×ÓŹżĪŖNAøö | |
| D£® | 1mol/L µÄNaOHČÜŅŗÖŠ£¬Na+µÄŹżÄæĪŖNA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 4ÖÖ | B£® | 5ÖÖ | C£® | 6ÖÖ | D£® | 7ÖÖ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ·“Ó¦ŗóÉś³ÉµÄŃĪÖ»ÓŠFe£ØNO3£©3 | |
| B£® | ·“Ó¦ŗóÉś³ÉµÄŃĪÖ»ÓŠFe£ØNO3£©2 | |
| C£® | ·“Ó¦ŗóÉś³ÉµÄŃĪĪŖFe£ØNO3£©3ŗĶFe£ØNO3£©2£¬ĘäĪļÖŹµÄĮæÖ®±ČĪŖ3£ŗ1 | |
| D£® | ·“Ó¦ŗóÉś³ÉµÄŃĪĪŖFe£ØNO3£©3ŗĶFe£ØNO3£©2£¬ĘäĪļÖŹµÄĮæÖ®±ČĪŖ1£ŗ3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| µĪ¶Ø“ĪŹż | “ż²āŅŗĢå»ż/mL | ±ź×¼ŃĪĖįĢå»ż/mL | |
| µĪ¶ØĒ°¶ĮŹż£ØmL£© | µĪ¶Øŗó¶ĮŹż£ØmL£© | ||
| µŚŅ»“Ī | 10.00 | 0.50 | 20.40 |
| µŚ¶ž“Ī | 10.00 | 4.00 | 24.10 |
| µŚČż“Ī | 10.00 | 4.20 | 25.70 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ŌŖĖŲ | I1 | I2 | I3 | I1+I2+I3 | I4 |
| Sc£ØīÖ£© | 633 | 1235 | 2389 | 4257 | 7019 |
| Y£ØīĘ£© | 616 | 1181 | 1980 | 3777 | 5963 |
| La£Øļē£© | 538 | 1067 | 1850 | 3455 | 4819 |
| Ce£Øīę£© | 527 | 1047 | 1949 | 3523 | 3547 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CH4+Cl2$\stackrel{¹ā}{”ś}$CH3Cl+HCl | |
| B£® |  +Br2$\stackrel{“߻ƼĮ}{”ś}$ +Br2$\stackrel{“߻ƼĮ}{”ś}$ +H2O +H2O | |
| C£® | 2CH3CH2OH+O2$”ś_{”÷}^{Cu»ņAg}$2CH3CHO+2H2O | |
| D£® | CH2ØTCH2+HCl”śCH3CH2Cl |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com