
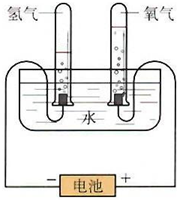
 ijѧϰС�����ͼ����ʾ�ĵ��ˮԭ������ʵ�飬����ʵ��õ�����������ϻ�õ����ݽ��д�������������������һ�����̽�����밴Ҫ����д��
ijѧϰС�����ͼ����ʾ�ĵ��ˮԭ������ʵ�飬����ʵ��õ�����������ϻ�õ����ݽ��д�������������������һ�����̽�����밴Ҫ����д��| ����g | ���ʵ���mol | H2 ��O2���ʵ����ı� | |
| H2 | |||
| O2 |
| ���� | ���� | 1mol���ʵ���� |
| 0��101kPa | H2 | 22.3L |
| O2 | 22.4L | |
| CO2 | 22.4L | |
| 25��101kPa | H2 | 24.4L |
| O2 | 24.5L | |
| CO2 | 24.5L |
| ||
| ||
| ||
| 0.2g |
| 2g/mol |
| 1.6g |
| 32g/mol |
| ����g | ���ʵ���mol | H2 ��O2���ʵ����ı� | |
| H2 | 0.2 | 0.1 | 2��1 |
| O2 | 1.6 | 0.05 |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �¶�/�� | 1 000 | 1 150 | 1 300 |
| ƽ�ⳣ�� | 64.0 | 50.7 | 42.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
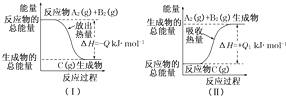
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
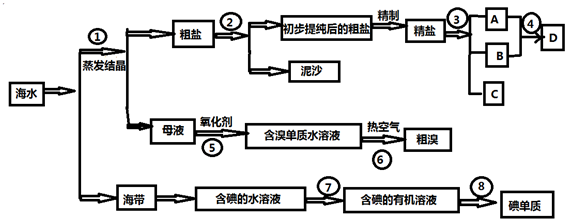
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
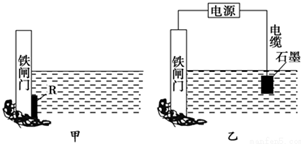
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��NaClO+CH3COOH�TCH3COONa+HClO | ||||
B��Cu2��OH��2CO3
| ||||
| C��2NO2+2NaOH�TNaNO3+NaNO2+H2O | ||||
| D��3CCl4+K2Cr2O7�T2CrO2Cl2+3COCl2+2KCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������H2��Cl2�����ķ�����һ���� |
| B��2mol?L-1��MgCl2��Һ�к�Mg2+��Ϊ2NA |
| C�����³�ѹ�£�11.2LCO2����������Ϊ0.5NA |
| D�������£�8g���飨CH4����������ԭ����Ϊ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Fe��ϡH2SO4��Ӧ�ų����壺Fe+6H+=2Fe3++3H2�� |
| B��ϡH2SO4��BaCl2��Һ��Ӧ���ɳ�����Ba2++SO42-�TBaSO4�� |
| C���ô���ʯ��ϡ�����Ʊ�CO2��CaCO3+2H+=Ca2++CO2��+H2O |
| D��̼������Һ������ϡ���CO32-+2H+=CO2��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
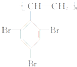 ����������������ȼ���������ֺϳɹ�����ͼ��ʾ��
����������������ȼ���������ֺϳɹ�����ͼ��ʾ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com