
����Ŀ������ع㷺Ӧ���ڻ�϶�������ϵͳ���缫������Ni(OH)2��̼�ۡ���������Ϳ�����������Ƴɡ����ڵ��ʹ�ú�缫���϶Ի�����Σ����ij��ȤС��Ըõ�ص缫���Ͻ�����Դ�����о������ʵ���������£�
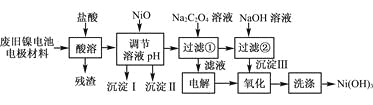
��֪����NiCl2������ˮ��Fe3����������Ni2����
����֪ʵ���¶�ʱ���ܽ�ȣ�NiC2O4��NiC2O4��H2O��NiC2O4��2H2O
��ij�¶���һЩ�������������Ksp����������������pH���±���ʾ��
|
| ��ʼ����pH | ������ȫpH |
|
|
|
|
|
|
|
|
|
|
|
|
�ش��������⣺
(1) ��NiO������Һ��pH����������������________�ͳ�����__________(�ѧʽ)��
(2) д������Na2C2O4��Һ�ķ�Ӧ�Ļ�ѧ����ʽ��_____________________��
(3) ��������Һʱ��������������ķ�����___________________________��
(4) д������������Ӧ�����ӷ���ʽ��___________________________________��
(5) ��μ���Ni(OH)3��ϴ�Ӹɾ���_______________________��
���𰸡�Fe(OH)3 Al(OH)3 NiCl2��Na2C2O4��2H2O=NiC2O4��2H2O����2NaCl ��ʪ��ĵ��۵⻯����ֽ 2Ni(OH)2��2OH����Cl2=2Ni(OH)3��2Cl�� ȡ���һ��ϴ��Һ������AgNO3��Һ�����а�ɫ�������ɣ������δϴ�Ӹɾ�����û�а�ɫ�������ɣ�֤��������ϴ�Ӹɾ�
��������
�Ͼ�����ص缫�����м�����������ʱ��Ni(OH)2��������������Ӧ����NiCl2��FeCl3��AlCl3������Ϊ̼�ۣ����ݱ������ݣ�����NiO������ҺpHʱ�����λ��Fe(OH)3��Al(OH)3��������֪ʵ���¶�ʱ���ܽ�ȣ�NiC2O4��NiC2O4��H2O��NiC2O42H2O������Һ�м���Na2C2O4���NiC2O4��2H2O��NaCl��NiC2O4��2H2O�м���NaOHת��ΪNi(OH)2�����NaCl��Һ�õ�NaOH��H2��Cl2�����Cl2��Ni(OH)2������Ni(OH)3���ݴ˷�������
�Ͼ�����ص缫�����м�����������ʱ��Ni(OH)2��������������Ӧ����NiCl2��FeCl3��AlCl3������Ϊ̼�ۣ����ݱ������ݣ�����NiO������ҺpHʱ�����λ��Fe(OH)3��Al(OH)3����������Һ�м���Na2C2O4���NiC2O4��2H2O��NaCl��NiC2O4��2H2O�м���NaOHת��ΪNi(OH)2�����NaCl��Һ�õ�NaOH��H2��Cl2�����Cl2��Ni(OH)2������Ni(OH)3��
��1�����ݱ��п�ʼ�����ͳ�����ȫ��pH��Fe��OH��3��ʼ������pH=2.5��������ȫ��pH=2.9��Al��OH��3��ʼ������pH=3.4��������ȫ��pH=4.2�������IΪFe��OH��3������IIΪAl��OH��3��
��2����֪ʵ���¶�ʱ���ܽ�ȣ�NiC2O4��NiC2O4��H2O��NiC2O42H2O����NiCl2��Һ�м���Na2C2O4��Ӧ����NiC2O42H2O��NaCl����Ӧ�ķ���ʽΪ��NiCl2+Na2C2O4+2H2O�TNiC2O42H2O��+2NaCl��
��3����ҺΪ�Ȼ�����Һ������Ȼ�����Һʱ����������������ʪ��ĵ��۵⻯����ֽ����Cl2��
��4�����ˢڵõ�Ni��OH��2������Ȼ�����Һ����������Cl2��Ni(OH)2������Ni(OH)3����Ӧ�����ӷ���ʽΪ2Ni��OH��2+2OH-+Cl2�T2Ni��OH��3+2Cl-��
��5���������̣�����Ni(OH)3��ϴ�Ӹɾ�ֻҪ����ϴ��Һ�в���Cl-���ɣ�����Ϊ��ȡ���һ��ϴ��Һ��������������Һ�����а�ɫ�������ɣ���˵��δϴ�Ӹɾ������ް�ɫ������˵����ϴ�Ӹɾ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ܱ�ʾH2(g)+ I2(g)![]() 2HI(g)�Ѿ��ﵽƽ��״̬�ı�־�м���( )
2HI(g)�Ѿ��ﵽƽ��״̬�ı�־�м���( )
��c(H2)=c(I2)=c(HI)ʱ��c(H2):c(I2):c(HI)=1:1:2ʱ��c(H2)��c(I2)��c(HI)������ʱ����ı�ܵ�λʱ��������nmolH2��ͬʱ����2nmolHI�ݵ�λʱ��������n mol H2��ͬʱ����n mol I2��Ӧ��v(H2)=v(I2)=1/2v(HI)��һ��H-H�����ѵ�ͬʱ������H-I�����Ѣ��¶Ⱥ����һ��ʱ��������ѹǿ���ٱ仯���¶Ⱥ����һ��ʱ������������ɫ���ٱ仯���¶Ⱥ�ѹǿһ��ʱ�����������ܶȲ��ٱ仯����һ������������ƽ����Է����������ٱ仯
A. 3B. 4C. 5D. 6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������A������Ԫ����ɣ�ij��ȤС�����������ʵ�飺

��֪�������Ϊ��������ֻ������Ԫ�أ��ڱ�������Ϊ672 mL����Һ��Ϊ������ҵ�г��õ�ճ�ϼ���
��ش��������⣺
��1��A�����Ԫ��Ϊ________����Ԫ�ط��ű�ʾ����
��2��д���������NaOH(aq)��Ӧ�����ӷ���ʽ________��
��3�������£�A�����������ܷ������ұ�ը���������ֳ�����������д����Ӧ�Ļ�ѧ����ʽ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ClO2��һ�����������������������Ƴ�NaClO2���壬�Ա���������棬�������ⷨ��NaClO2�����ʵ��װ����ͼ��ʾ��
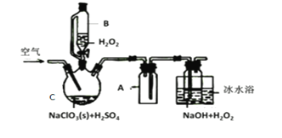
��֪����2NaC1O3+H2O2+H2SO4=2C1O2��+O2��+Na2SO4+2H2O
2ClO2+H2O2+2NaOH=2NaClO2+O2��+2H2O
��ClO2�۵�-59�桢�е�11�棬Ũ�ȹ���ʱ�����ֽ⣻
��H2O2�е�150��
��1����ˮԡ��ȴ��Ŀ����___��
��2���������ٹ���������������NaClO2���ʣ��Խ�����ԭ�������ٹ���ʱ��__��
��3��Cl-����ʱ���ClO2�����ɡ���Ӧ��ʼʱ��C�м����������ᣬClO2���������ʴ����ߣ����������������ù��̿��ܾ�������ɣ��뽫�䲹��������
��___�������ӷ���ʽ��ʾ����H2O2+Cl2=2Cl-+O2+2H+
��4��NaClO2���Ȳⶨ��
��ȷ��ȡ����NaClO2��Ʒ10.0g���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ��C1O2-�IJ���ΪCl-���������û��Һ���250mL������Һ��
��ȡ25.00mL����Һ����2.0mol��L-1Na2S2O3��Һ�ζ�(I2+2S2O32-=2I-+S4O62-)���Ե�����Һ��ָʾ�����ﵽ�ζ��յ�ʱ������Ϊ__���ظ��ζ�3�Σ����Na2S2O3��Һƽ������Ϊ20.00mL�������Ʒ��NaClO2����������Ϊ___����M(NaClO2)=90.5g/mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�������ĵ���ƽ�ⳣ�����£�
��ѧʽ | HF | HCN | H2CO3 |
���볣�� | Ka=3.5��10-4 | Ka=5.0��10-10 | Ka1=4.4��10-7 Ka2=4.7��10-11 |
��1��c(H+)��ͬ����������Һ��Ũ�ȴӴ�СΪ___��
��2����HCN��Һ����ʼŨ��Ϊ0.01mol��L-1��ƽ��ʱc(H+)ԼΪ__mol��L-1��ʹ����Һ��HCN�ĵ���̶�������c(H+)Ҳ����ķ�����__��
��3���к͵�����NaOH�����ĵ�pH�����������������ֱ�ΪaL��bL����a__(������������С������������������ͬ)b���к͵�Ũ�ȡ��������������������ҪNaOH�����ʵ���Ϊn1��n2����n1__n2��
��4����NaCN��Һ��ͨ��������CO2��������Ӧ�����ӷ���ʽΪ__��
��5�����ʵ��֤��������HCl��������__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����(N2H4)�ǻ����������ȼ�ϣ�����N2O4��Ӧʱ��N2O4Ϊ�����������ɵ�����ˮ��������֪����N2(g)��2O2(g)��N2O4(g)��H����8.7kJ��mol��1����N2H4(g)��O2(g)��N2(g)��2H2O(g)��H����534.0kJ��mol��1�����б�ʾ�¸�N2O4��Ӧ���Ȼ�ѧ����ʽ��ȷ����
A.2N2H4(g)��N2O4(g)��3N2(g)��4H2O(g)��H=��542.7kJ��mol��1
B.2N2H4(g)��N2O4(g)��3N2(g)��4H2O(g)��H=��1059.3kJ��mol��1
C.N2H4(g)��![]() N2O4(g)��
N2O4(g)��![]() N2(g)��2H2O(g)��H=��1076.7kJ��mol��1
N2(g)��2H2O(g)��H=��1076.7kJ��mol��1
D.2N2H4(g)��N2O4(g)��3N2(g)��4H2O(g)��H=��1076.7kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������X������Ԫ����ɣ�Ϊ̽������ɵ����ʣ���Ʋ��������ʵ�飺
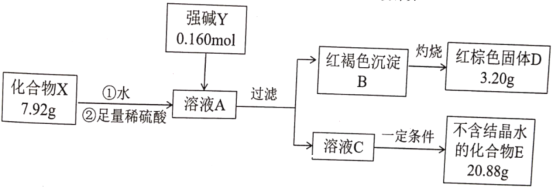
��ʾ��������E����ɫ��ӦΪ��ɫ������ɫ�ܲ�����
��ش�
��1��X�Ļ�ѧʽ��________________��ǿ��Y�ĵ���ʽΪ________________��
��2���ڳ��º���������£�������X�����ȶ����ڣ�������ˮ��Һ�в��ȶ���һ��ʱ���ת��Ϊ���ɫ������һ�����嵥�ʡ�
�ٻ�����X��ˮ��Ӧ�����ӷ���ʽΪ________________��
��������Ի�����X���ȶ��Խ����˴������о�����ȡ����һ���Ľ�չ���������ʿ����������X��ˮ��Һ���ȶ��Ե���________________��
A KHSO4 B K2CO3 C CH3COOK D K2SO3
��Ϊ�о��¶ȶԻ�����Xˮ��Һ�ȶ��Ե�Ӱ�죬�����һ��ʵ�鷽����________________________________________________��
��3��������X�ж����Ʊ���������һ�ַ�������ǿ��Y�������ô����������ɫ����B��Ӧ���仯ѧ����ʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ӵ�������ֵΪNA������˵����ȷ���ǣ� ��
A.1 L 0.1 mol��![]() NH4Cl��Һ�У�NH4+������Ϊ0.1NA
NH4Cl��Һ�У�NH4+������Ϊ0.1NA
B.2.4gþ��������O2��ȼ�գ�ת�Ƶĵ�����Ϊ0.1NA
C.��״���£�5.6 L CO2�����к��е���ԭ����Ϊ0.5NA
D.1 mol N2��3 mol H2��Ӧ���ɵ�NH3������Ϊ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Cl2O�ǻ���ɫ����ǿ�Ҵ̼�����ζ�����壬��һ��ǿ��������������ˮ�һ���ˮ��Ӧ���ɴ����ᣬ���л����ԭ���Ӵ������ʱ�ᷢ��ȼ�ղ���ը����ȡCl2O��װ����ͼ��ʾ��

��֪��Cl2O���۵�Ϊ��116 �棬�е�Ϊ3.8 �棬Cl2O�ڿ����еİٷֺ����ﵽ25�������ױ�ը��Cl2�ķе�Ϊ��34.6 �棻HgO��2Cl2==HgCl2��Cl2O������˵���в���ȷ����
A.װ�âڢ���ʢװ���Լ������DZ���ʳ��ˮ��Ũ����
B.ͨ����������Ŀ���ǽ����ɵ�Cl2Oϡ�ͣ���С��ըΣ��
C.��װ�â����ݳ��������Ҫ�ɷ���Cl2O
D.װ�â����֮�䲻�������ӣ���Ϊ�˷�ֹ��ȼ�պͱ�ը
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com