
ijЩ»¯Ñ§¼üµÄ¼üÄÜÈçϱíËùʾ£¨kJ?mol£1£©£º
¼ü | H£H | Br£Br | I£I | Cl£Cl | H£Cl | H£I | H£Br |
¼üÄÜ | 436 | 193 | 151 | 247 | 431 | 299 | 356 |
£¨1£©°Ñ1mol Cl2·Ö½âΪÆø̬Ô×Óʱ£¬ÐèÒª £¨Ìî¡°ÎüÊÕ¡±»ò¡°·Å³ö¡±£© ÄÜÁ¿
£¨2£©1mol H2 ÔÚ2mol Cl2ÖÐȼÉÕ£¬·Å³öµÄÈÈÁ¿ KJ¡£
£¨3£©ÓɱíÖÐËùÁл¯Ñ§¼üÐγɵĵ¥ÖÊ·Ö×ÓÖУ¬×îÎȶ¨µÄÊÇ £¬×î²»Îȶ¨µÄÊÇ £¬ÐγɵĻ¯ºÏÎï·Ö×ÓÖУ¬×îÎȶ¨µÄÊÇ £¬×î²»Îȶ¨µÄÊÇ £¬
£¨4£©ÔÚÒ»¶¨Ìõ¼þÏ£¬1mol H2 Óë×ãÁ¿µÄCl2 ¡¢Br2 ¡¢I2 ·Ö±ð·´Ó¦£¬·Å³öÈÈÁ¿ÓɶൽÉÙµÄÊÇ______ __¡£
A£®Cl2 > Br2 > I2 B£®I2 > Br2 > Cl2
£¨5£©Ô¤²â1mol H2 ÔÚ×ãÁ¿F2 ÖÐȼÉÕ±ÈÔÚCl2ÖзÅÈÈ ¡£

| Ä꼶 | ¸ßÖÐ¿Î³Ì | Ä꼶 | ³õÖÐ¿Î³Ì |
| ¸ßÒ» | ¸ßÒ»Ãâ·Ñ¿Î³ÌÍƼö£¡ | ³õÒ» | ³õÒ»Ãâ·Ñ¿Î³ÌÍƼö£¡ |
| ¸ß¶þ | ¸ß¶þÃâ·Ñ¿Î³ÌÍƼö£¡ | ³õ¶þ | ³õ¶þÃâ·Ñ¿Î³ÌÍƼö£¡ |
| ¸ßÈý | ¸ßÈýÃâ·Ñ¿Î³ÌÍƼö£¡ | ³õÈý | ³õÈýÃâ·Ñ¿Î³ÌÍƼö£¡ |
¿ÆÄ¿£º¸ßÖл¯Ñ§ À´Ô´£º ÌâÐÍ£º
| ¼ü | H-H | Br-Br | I-I | Cl-Cl | H-Cl | H-I | H-Br |
| ¼üÄÜ | 436 | 193 | 151 | 247 | 431 | 299 | 356 |
²é¿´´ð°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖл¯Ñ§ À´Ô´£º ÌâÐÍ£º
¼ü | H¡ªH | Br¡ªBr | I¡ªI | Cl¡ªCl | H¡ªCl | H¡ªI | H¡ªBr |
¼üÄÜ | 193 | 436 | 151 | 247 | 431 | 299 | 356 |
£¨1£©1 mol H2ÔÚ2 mol Cl2ÖÐȼÉÕ£¬·Å³öÈÈÁ¿____________kJ¡£
£¨2£©ÔÚÒ»¶¨Ìõ¼þÏ£¬1 mol H2Óë×ãÁ¿µÄCl2¡¢Br2¡¢I2·Ö±ð·´Ó¦£¬·Å³öÈÈÁ¿ÓɶൽÉÙµÄÊÇ__________¡£
A.Cl2£¾Br2£¾I2 B.I2£¾Br2£¾Cl2
Ô¤²â1 mol H2ÔÚ×ãÁ¿F2ÖÐȼÉÕ±ÈÔÚCl2ÖзÅÈÈ____________¡£
²é¿´´ð°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖл¯Ñ§ À´Ô´£º ÌâÐÍ£º
¼ü | H¡ªH | Br¡ªBr | I¡ªI | Cl¡ªCl | H¡ªCl | H¡ªI | H¡ªBr |
¼üÄÜ | 193 | 436 | 151 | 247 | 431 | 299 | 356 |
£¨1£©1 mol H2ÔÚ2 mol Cl2ÖÐȼÉÕ£¬·Å³öÈÈÁ¿________________kJ¡£
£¨2£©ÔÚÒ»¶¨Ìõ¼þÏ£¬1 mol H2Óë×ãÁ¿µÄCl2¡¢Br2¡¢I2·Ö±ð·´Ó¦£¬·Å³öÈÈÁ¿ÓɶൽÉÙµÄ˳ÐòÊÇ________________¡£
a.Cl2£¾Br2£¾I2 b.I2£¾Br2£¾Cl2
Ô¤²â1 mol H2ÔÚ×ãÁ¿F2ÖÐȼÉÕ±ÈÔÚCl2ÖзÅÈÈ________________¡£
²é¿´´ð°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖл¯Ñ§ À´Ô´£º2012-2013ѧÄêËÄ´¨Ê¡Äϳä¸ßÖи߶þÉÏѧÆÚÆÚÖп¼ÊÔÎÄ¿Æ»¯Ñ§ÊÔ¾í£¨´ø½âÎö£© ÌâÐÍ£ºÌî¿ÕÌâ
ijЩ»¯Ñ§¼üµÄ¼üÄÜÈçϱíËùʾ£¨kJ¡¤mol£1£©£º
| ¼ü | H£H | Br£Br | I£I | Cl£Cl | H£Cl | H£I | H£Br |
| ¼üÄÜ | 436 | 193 | 151 | 247 | 431 | 299 | 356 |
²é¿´´ð°¸ºÍ½âÎö>>
¿ÆÄ¿£º¸ßÖл¯Ñ§ À´Ô´£º2013½ìÕã½Ê¡ÎÂÖÝÊÐʮУÁªºÏÌå¸ßÈýÉÏѧÆÚÆÚ³õ¿¼ÊÔ»¯Ñ§ÊÔ¾í£¨´ø½âÎö£© ÌâÐÍ£ºÌî¿ÕÌâ
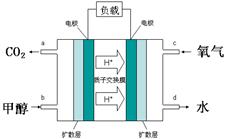
£¨12·Ö£©¼×´¼ÊÇÒ»ÖÖÐÂÐ͵ÄÆû³µ¶¯Á¦È¼ÁÏ£¬¹¤ÒµÉÏ¿Éͨ¹ýCOºÍH2»¯ºÏÖƱ¸¼×´¼£¬¸Ã·´Ó¦µÄÈÈ»¯Ñ§·½³ÌʽΪ£ºCO(g)+2H2(g) CH3OH(g) ¨SH ÒÑ֪ijЩ»¯Ñ§¼üµÄ¼üÄÜÊý¾ÝÈçÏÂ±í£º
CH3OH(g) ¨SH ÒÑ֪ijЩ»¯Ñ§¼üµÄ¼üÄÜÊý¾ÝÈçÏÂ±í£º
| »¯Ñ§¼ü | C¡ªC | C¡ªH | H¡ªH | C¡ªO | C¡ÔO | H¡ªO |
| ¼üÄÜ/kJ¡¤mol-1 | 348 | 413 | 436 | 358 | 1072 | 463 |
| ·´Ó¦Ê±¼ä/min | 0 | 5 | 10 | 15 | 20 | 25 |
| ѹǿ/MPa | 12.6 | 10.8 | 9.5 | 8.7 | 8.4 | 8.4 |
²é¿´´ð°¸ºÍ½âÎö>>
°Ù¶ÈÖÂÐÅ - Á·Ï°²áÁбí - ÊÔÌâÁбí
ºþ±±Ê¡»¥ÁªÍøÎ¥·¨ºÍ²»Á¼ÐÅÏ¢¾Ù±¨Æ½Ì¨ | ÍøÉÏÓк¦ÐÅÏ¢¾Ù±¨×¨Çø | µçÐÅթƾٱ¨×¨Çø | ÉæÀúÊ·ÐéÎÞÖ÷ÒåÓк¦ÐÅÏ¢¾Ù±¨×¨Çø | ÉæÆóÇÖȨ¾Ù±¨×¨Çø
Î¥·¨ºÍ²»Á¼ÐÅÏ¢¾Ù±¨µç»°£º027-86699610 ¾Ù±¨ÓÊÏ䣺58377363@163.com