
�ҹ��ӰĴ����ǽ��ڵ�ij��¯��������A��ʾ���ijɷ����£�������������
| C | Si | Mn | P | S |
| 4.070% | 2.900% | 0.495% | 0.165% | 0.010% |
| | FeO | Fe2O3 | CaO |
| �̳�����ǰ��%�� | 86.40 | 4.00 | 9.60 |
��1��Mn3O4��2�֣�
��2�� 27.46
��3��132.701��3�֣�
��4����Ӧ����������� n(Fe):n(O)=1��1.4
������FeO��Fe2O3�Ļ��������ǵ����ʵ���֮��Ϊ1��2��
������Fe3O4��Fe2O3�Ļ��������ǵ����ʵ���֮��Ϊ1��1��
���������������1��100.000g A����Mn������=100.000g��0.495%=0.495g������MnxOY��OԪ�ص�����=0.687g-0.495g=0.192g����x��y="0.495/55" :0.192/16=3��4���ʸ�������Ļ�ѧʽΪMn3O4���ʴ�Ϊ��Mn3O4��
��2����Ca��H2PO4��2��дCaH4O3?P2O5����P2O5�����������T45.25%��142/234=27.46%���ո��л����ܻ���Ca3��PO4��2��������P2O5��ʾ���������������ܴ���27.46%����P2O5������������27.46%���ʴ�Ϊ����27.46%��
��3����������ɸ��̸�y�֣�����FeԪ���������䣬��y����1-30%-0.4%��=100����1-4.070%-2.9%-0.495%-0.165%-0.010%����ã�y=132.701���ʴ�Ϊ��132.701��
��4�������̳�����ǰ�̳�������Ϊ100g����n��FeO��=1.2mol��n��Fe2O3��=0.025mol��FeԪ�����ʵ���=1.2mol+0.05mol=1.25mol��������=1.25mol��56g/mol=70g���̳�����ǰ��FeԪ�ص��������䣮���պ�CaO������Ҳ���䣬���պ����ʵ�����=100*9.6%/8.92%=107.6g������OԪ�ص�����=107.6g����1-8.92%��-70g=28.0g��n��O��=28/16=1.75mol����Ӧ����������� n��Fe����n��O��=1.25mol��1.75mol=1��1.4�����̳����պ������������ƽ�����ΪFeO1.4�����ij�����������FeO��Fe2O3��Fe3O4��������FeO��Fe2O3�Ļ�����FeO��Fe2O3�ķֱ�Ϊxmol��ymol����(X+3Y)/(X+2Y)=1.4��������x��y=1��2����FeO��Fe2O3�����ʵ���֮��Ϊ1��2��������Fe3O4��Fe2O3�Ļ�����Fe3O4��Fe2O3��xmol��ymol����(4X+3Y)/(3X+2Y)=1.4��������x��y=1��1����Fe3O4��Fe2O3�����ʵ���֮��Ϊ1��1��������FeO��Fe3O4�Ļ���������ƽ����ɣ���������������FeO��Fe2O3��Fe3O4�Ļ������ü����жϣ���FeO��Fe2O3�Ļ���Fe2O3�ĺ������Ϊ2/3=66.7%����ΪFe3O4��Fe2O3�Ļ���Fe2O3�ĺ������Ϊ1/2=50%����50%��Fe2O3�����ʵ���������66.7%���𣺢�����FeO��Fe2O3�Ļ��������ǵ����ʵ���֮��Ϊ1��2��������Fe3O4��Fe2O3�Ļ��������ǵ����ʵ���֮��Ϊ1��1��������FeO��Fe3O4�Ļ�����������������FeO��Fe2O3��Fe3O4�Ļ���50%��Fe2O3�����ʵ���������66.7%��
���㣺����������Ϊ���壬�������ʵ������ؼ��㼰ƽ��ֵ����Ӧ�ã���Ŀ�ѶȽϴ��Ƕ�ѧ���ۺ������Ŀ��顣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ҵĻ������÷����ܶ࣬���ú���Al2O3��SiO2������FeO xFe2O3�������Ʊ�Al2(S04)3
xFe2O3�������Ʊ�Al2(S04)3 18H2O�������������£�
18H2O�������������£�
��ش��������⣺
��1���������ϡH2SO4�ܽ�Al2O3�����ӷ���ʽ��______________��
��2�������м��˵�KMnO4Ҳ����H2O2���棬����H2O2������Ӧ�Ļ�ѧ����ʽΪ_______________��
��3����֪��Ũ�Ⱦ�ΪO.1mol/L�Ľ��������ӣ������������������pH���±���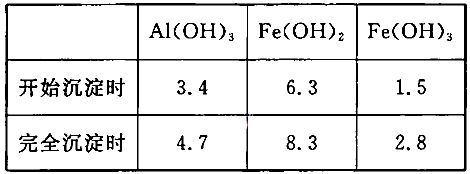
����۵�Ŀ����__________________________________________________________�����ڸ�Ũ���³�ȥ���Ļ��������pH�����Χ��___________��
��4����֪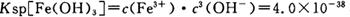 ��pH=2ʱ��Fe3����ʼ������Ũ��Ϊ_______________��
��pH=2ʱ��Fe3����ʼ������Ũ��Ϊ_______________��
��5�������ܷ�����Ӧ�����ӷ���ʽΪ__________________________________________��Ϊ����֤�ò������ù�����ȷʵ����MnO2����ѡ�õ��Լ���_________��_________��
��6�������ݡ�һϵ�в���"�����������в����õ���___________������ţ���
| A�������� | B������ | C�������� | D���ƾ���E��©�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��20 mL ijŨ�ȵ�AlCl3��Һ�е���2 mol��L��1��NaOH��Һʱ�����õij������������NaOH��Һ�����֮��Ĺ�ϵ��ͼ��ʾ��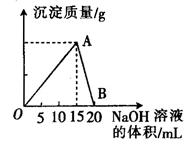
��1��ͼ��A���ʾ��������______________��
��2������������______________g��
��3��B���ʾ��������______________��
��4������AlCl3��Һ�����ʵ���Ũ����______________��
��5�������ó�����Ϊ0.39��ʱ����ȥNaOH��Һ�������_____ mL ��_______ mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���Ѱ۲����ķ�Һ�к��д���FeSO4��H2SO4������Fe2(SO4)3��TiOSO4����������ⷨ������Ѫ�������������������������£�
��֪��TiOSO4������ˮ����ˮ�е���ΪTiO2+��SO42-����ش��������⣺
��1��д��TiOSO4ˮ����������H4TiO4�����ӷ���ʽ ��������м���������м��Ŀ���� ��
��2����ҵ����H4TiO4���Ƶ��Ѱ�TiO2��TiO2ֱ�ӵ�ԭ�������ŷ��������� ��һ�ֽ��Ƚ��ķ����������Ϊ���ڵ�CaCl2��ԭ����ͼ��ʾ�������ĵ缫��ӦΪ_______________��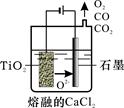
��3������ڵ����ӷ���ʽ�� �����ø���Ʒ��Ҫ ��__________���ѧʽ����
��4������ܵĽᾧ�����б������һ������նȣ�ԭ���� ��
��5�����������ϩ�����в���ϳɣ�
�����ϳ�·�ߵ��ܲ���Ϊ60%��������̼��������Ӧת��Ϊ������������IJ���Ϊ90%��������468 kg�����������壨M��234 g/mol����Ҫ��״���µ���ϩ m3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͭ������ϡ���ᣬ��������������Һ���ֽ�һ������ͭƬ���뵽100 mLϡ������������Ļ����Һ�У�ͭƬ��ȫ�ܽ⣨�������ε�ˮ�⼰��Һ����ı仯����
��1��д��ͭ�ܽ������������Һ�����ӷ���ʽ_______________________________________��
��2����ͭ��ȫ�ܽ�ʱ����Һ�е�Fe3����Cu2����H���������ӵ����ʵ���Ũ����ȣ��Ҳ����Һ��pH��1�����ܽ�ͭ��������_________g����Һ�е�c(SO42��)��__________mol/L��
��3����������ͼ��ʾ��װ���з�����1���еķ�Ӧ����X���� ���������������缫��Ӧʽ ��Y���IJ����� ���缫��Ӧʽ ��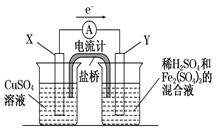
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ú���Al2O3������Fe2O3��SiO2���������Ʊ���ˮ������Һ��ۺ����������������������£����ֲ����������ԣ���
I�����������м������H2SO4���ȡ����衢���ˡ�
II������Һ�м���һ������FeSO4��7H2O��˫��ˮ��
III������Һ�м���Ca(OH)2���壬������Һ��pHԼΪ1�����ˡ�
IV�������ȶ��������ȣ��õ���Ʒ��
��1��Fe2O3��H2SO4��Ӧ�����ӷ���ʽ��___________��
��2������I�й��˵õ��������ɷ���________���ѧʽ����
��3������I ��H2SO4��Ũ���뷴Ӧ�¶Ȼ�Ӱ���������Ľ����ʡ�������ͼ����������I ��H2SO4Ũ�ȵ����˷�Χ��__________����Ӧ�������¶���_________��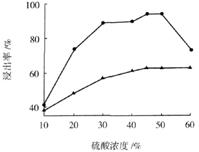

��4������II������n(Fe3+)�����ӷ���ʽ��_________��
��5������III�õ���ʽ��������[AlFe(OH)n(SO4)m]����Һ������II��Ӧ����n(Fe3+)��
n(Al3+)�sn(Fe3+)= ��
��6���о�������Һ��ۺ����������Ĵ���Խ�ߣ���ˮЧ��Խ�á���֪��
һЩ������20��ʱ���ܽ��
| ���� | Ca(OH)2 | CaSO4 | Na2SO4 |
| �ܽ��/g | 0.153 | 0.258 | 19.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���к�FeCl2���ʵ��Ȼ�ͭ���壨CuCl2��2H2O����Ϊ��ȡ������CuCl2��2H2O�����Ƚ����Ƴ�ˮ��Һ��Ȼ����ͼ��ʾ��������ᴿ��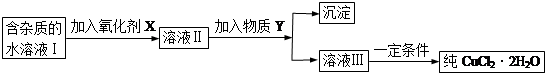

��֪H2O2��KMnO4��NaClO��K2Cr2O7������ǿ�����ԣ�Ҫ����Һ�е�Cu2����Fe2����Fe3������Ϊ�����������Һ��pH�ֱ�Ϊ6.4��6.4��3.7��
��ش��������� [��1��~��2��С������] ��
��1����ʵ�����ʺϵ�������X��__________
A��K2Cr2O7 B��NaClO C��H2O2 D��KMnO4
��2������Y��������___________
A��CuO B��CuCl2 C��Cu��OH��2 D��CuCO3
��3����YΪCu(OH)2��д����ȥFe3�������ӷ���ʽ��
��4��������������Ŀ����_______________________________________��
��5������ܲ���ֱ�������ᾧ�õ�CuCl2��2H2O��__________����ܡ����ܡ������粻�ܣ�Ӧ��β����������ܣ��˿ղ��_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�й����ʼ�ת����ϵ����ͼ���Իش�
��1��ת���ٵķ�Ӧ����Ϊ .
��2��ת���ڵĻ�ѧ����ʽΪ .ת���۵����ӷ���ʽΪ .
��3����ת��������ʵ������Al(OH)3�����Լ�A��ѡ�� �������ƣ�.
��4����50 mL 3 mol��L-1 AlCl3��Һ�еμ�1 mol��L-1 NaOH��Һ�����Al3+������1/3ת��ΪAl(OH) 3������������NaOH��Һ���������Ϊ mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������п��������������ã�������ҩ����ˮ���ȣ�4.74g����������[KAl(SO4)2��12H2O]������ˮ�Ĺ����е���������m~n(��Ʒ���������¶ȱ仯������)���¡�
��ش��������⣺
��1����ȷ��70��ʱ�������ʵĻ�ѧʽ____________________ (Ҫ��д���ƶϹ���)��
��2��д��200��ʱ������Ӧ�Ļ�ѧ����ʽ______________________________��
��3����770��ʱ������գ����õ��������A������B��B��ȫ���ܽ���BaCl2��Һ�в��γɰ�ɫ������A�ɲ�������ˮ�����˺�õ�����C����ҺD����D�м���BaCl2��ҺҲ�ܵõ���ɫ��������C����������NaOH��Һ��ǡ�÷�Ӧ�õ��������ҺE����n�й���ɷ��ǵ�_________�������ֱ�Ϊ__________B�Ļ�ѧʽΪ________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com