
ʵ������ȼ�շ��ⶨij�ְ�����(CxHyOzNp)�ķ�����ɡ�ȡw g���ְ�������ڴ����г��ȼ�գ�����CO2��H2O��N2��������ͼ��ʾװ�ý���ʵ�飨����̨�����С��ƾ��Ƶ�δ����������ش��й����⣺
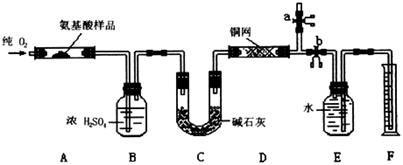
��1��ʵ�鿪ʼʱ�����ȴ�ֹˮ��a���ر�ֹˮ��b��ͨһ��ʱ��Ĵ�������������Ŀ���� ��֮������ر�ֹˮ�� ����ֹˮ�� ��
��2������װ������Ҫ���ȵ��У���װ�ô��ţ� ������ʱӦ�ȵ�ȼ ���ľƾ��ơ�
��3��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��4��װ��D�������� ��
��5��Ϊ��N2����ˮ�����������ӦǰӦ������E��Fװ�õIJ��������г��� ��ˮ������������������������ ��
��6����ȡN2����ˮ�����ʱ��Ҫע�⣺�� ��
�� ��
��7��ʵ���в��N2�����ΪV mL���ѻ���ɱ�״������Ϊȷ���˰�����ķ���ʽ������õ��������У�����ĸ�� ��
A.���ɶ�����̼��������� B.����ˮ������
C.ͨ����������� D.�ð������Ħ������
��8�������װ����B��C������˳���ΪC��B����ʵ���Ŀ���ܷ�ﵽ���������� ��
��1����װ���е� �ž���a��b����2��A��D��D
�ž���a��b����2��A��D��D
��3��
��4������δ��Ӧ��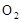 ����֤�����ռ����������Ƿ�Ӧ���ɵ�
����֤�����ռ����������Ƿ�Ӧ���ɵ�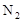 ��
��
��5��ˮ����β���������������壬�����ܱ��ռ��Ͳ��������½ϴ��ʵ����������ˮʱ����֤����װ��E������������ų�ˮ�����������ȡ�
��6������Ͳ�е�Һ��Ӧ����ƿ�е�Һ�����ƽ��������Ӧ��̶��ߺͰ�Һ����͵����С�
��7��A��B��D
��8�����ܴﵽ��ʵ���Ŀ�ġ���Ϊ��ʯ���Ǽ��Ը��������ͬʱ����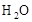 ��
��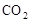 �������壬ʹʵ����ȱ�ٱ�Ҫ�����ݣ���ȷ���ð�����ķ�����ɡ�
�������壬ʹʵ����ȱ�ٱ�Ҫ�����ݣ���ȷ���ð�����ķ�����ɡ�
��������
�����������1������װ���к��п������������к��е����������ʵ�顣����Ŀ�ľ��ǽ�װ���е�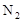 �ž�����ֹ���ţ����ʵ����Ȼ��Ϳ��Թر�ֹˮ��a����ֹˮ��b��
�ž�����ֹ���ţ����ʵ����Ȼ��Ϳ��Թر�ֹˮ��a����ֹˮ��b��
��2��������������ķ�Ӧ���Լ�ͭ���������ķ�Ӧ����Ҫ���ȣ���ѡA��D��Ϊ�˷�ֹ���������ս���E�У���������Ҫ��ȼD���ƾ��ơ�
��3���������������Ӧ�Ļ�ѧ����ʽ��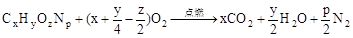 ��
��
��4���������Ϸ�����֪��ͭ��������������δ��Ӧ��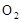 ����֤�����ռ����������Ƿ�Ӧ���ɵ�
����֤�����ռ����������Ƿ�Ӧ���ɵ�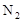 ��
��
��5����β���������������壬�����ܱ��ռ��Ͳ��������½ϴ��ʵ����������ˮʱ����֤����װ��E������������ų�ˮ�����������ȣ�����Ϊ��N2����ˮ�����������ӦǰӦ������E��Fװ�õIJ��������г���ˮ��
��6������ʱ���뱣֤ѹǿ��ͬ������ע�������Ǣ���Ͳ�е�Һ��Ӧ����ƿ�е�Һ�����ƽ���������Ӧ��̶��ߺͰ�Һ����͵����С�
��7������ͨ����������ֱ�ͭ��Ӧ���������������������Ҫ�����ݷ�Ӧ�Ļ�ѧ����ʽ��֪��Ҫ�ⶨ������Ļ�ѧʽ������������غ㶨�ɿ�֪������Ҫ�����������ɶ�����̼���������������ˮ�������Լ��ð������Ħ����������ѡA��B��D��
��8����Ϊ��ʯ���Ǽ��Ը��������ͬʱ����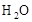 ��
��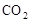 �������壬ʹʵ����ȱ�ٱ�Ҫ�����ݣ���ȷ���ð�����ķ�����ɣ����Բ��ܶԵ���
�������壬ʹʵ����ȱ�ٱ�Ҫ�����ݣ���ȷ���ð�����ķ�����ɣ����Բ��ܶԵ���
���㣺���鰱���ữѧʽ�ⶨ���й�ʵ���жϡ�ʵ�����ۡ�����Լ�ʵ�����ݴ�����
�����������Ǹ߿��еij������ͣ������ۺ���ǿ���ѵ�ϴ�ѧ�����ۺ���������˸��ߵ�Ҫ��������Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۡ���������������ѧ������˼ά�����ͷ�ɢ˼ά�������ر���ѧ����ʵ����ơ����������۵��ۺ�������Ҳ����������ѧ����ѧϰ��Ȥ�����ѧ����ѧϰЧ�ʡ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

������������⣺
(1)ʵ�鿪ʼʱ������Ҫͨ��һ��ʱ�����������������______________________��
(2)����װ������Ҫ���ȵ�������______________________(����ĸ��գ���ͬ)������ʱӦ�ȵ�ȼ__________���ľƾ��ơ�
(3)Aװ���з�����Ӧ�Ļ�ѧ����ʽ��____________________________��
(4)װ��D��������_____________________________��
(5)��ȡN2�����ʱ��Ӧע���______________________����______________________��
(6)ʵ���в��N2�����ΪV mL(��״��)��Ϊȷ���˰�����Ļ�ѧʽ������Ҫ���й�������_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��������ѧ��һDZ�ܰ�����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(14��)ʵ������ȼ�շ��ⶨij�����л���A�ķ�����ɣ��ⶨװ����ͼ(����̨�����С��ƾ��Ƶ�δ����)��
ȡ17.1 g A����װ���У�ͨ�����O2ȼ�գ�����CO2��H2O����ش������й����⣺
(1)ͨ�����O2��Ŀ����______________________________________________��
(2)Cװ�õ�������__________________________________________��
Dװ�õ�������_____________________________________________��
(3)ͨ����ʵ�飬�ܷ�ȷ��A���Ƿ�����ԭ�ӣ�________��
(4)��A��Ħ������Ϊ342 g/mol��Cװ������9.99 g��Dװ������26.4 g����A����ʽΪ____________��
(5)д��Aȼ�յĻ�ѧ����ʽ_____________________________________��
(6)A�ɷ���ˮ�ⷴӦ��1 mol A��ˮ������2 molͬ���칹�壬��A�ڴ���������ˮ��Ļ�ѧ����ʽΪ_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��������ѧ��һDZ�ܰ�����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
(14��)ʵ������ȼ�շ��ⶨij�����л���A�ķ�����ɣ��ⶨװ����ͼ(����̨�����С��ƾ��Ƶ�δ����)��

ȡ17.1 g A����װ���У�ͨ�����O2ȼ�գ�����CO2��H2O����ش������й����⣺
(1)ͨ�����O2��Ŀ����______________________________________________��
(2)Cװ�õ�������__________________________________________��
Dװ�õ�������_____________________________________________��
(3)ͨ����ʵ�飬�ܷ�ȷ��A���Ƿ�����ԭ�ӣ�________��
(4)��A��Ħ������Ϊ342 g/mol��Cװ������9.99 g��Dװ������26.4 g����A����ʽΪ____________��
(5)д��Aȼ�յĻ�ѧ����ʽ_____________________________________��
(6)A�ɷ���ˮ�ⷴӦ��1 mol A��ˮ������2 molͬ���칹�壬��A�ڴ���������ˮ��Ļ�ѧ����ʽΪ_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������ȼ�շ��ⶨij������CxHyOzNp�ķ�����ɡ�ȡmg���ְ�������ڴ����г��ȼ�գ�����CO2��H2O��N2��ʵ��װ������ͼ��

��ش������й����⣺
��1��ʵ�鿪ʼʱ������Ҫͨ��һ��ʱ����������������ǣ�
��
��2������װ������Ҫ���ȵ������� ������ĸ����ͬ������ʱӦ�ȵ�ȼ
���ľƾ��ơ�
��3��Aװ���з�����Ӧ�Ļ�ѧ����ʽ�ǣ�
��4��װ��D�������ǣ�
��5����ȡN2���ʱ��Ӧע�⣺�� ��
�� ��
��6��ʵ���в��N2�����VmL��������Ϊ��״������Ϊȷ���˰�����ķ���ʽ������Ҫ���й������� ������ĸ��գ���
A�����ɶ�����̼���������
B�����������Է�������
C��ͨ�����������
D������ˮ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com