
ij¹¤Ņµ·ĻĖ®½öŗ¬ĻĀ±ķÖŠµÄijŠ©Ąė×Ó£¬ĒŅø÷ÖÖĄė×ÓµÄĪļÖŹµÄĮæÅضČĻąµČ£¬¾łĪŖ0.1 mol/L(“ĖŹżÖµŗöĀŌĖ®µÄµēĄė¼°Ąė×ÓµÄĖ®½ā)”£
| ŃōĄė×Ó | K£« | Ag£« | Mg2£« | Cu2£« | Al3£« | NH |
| ŅõĄė×Ó | Cl£ | CO | NO | SO | SiO | I£ |
¼×Ķ¬Ń§ÓūĢ½¾æ·ĻĖ®µÄ×é³É£¬½ųŠŠĮĖČēĻĀŹµŃé£ŗ
¢ń.Č”øĆĪŽÉ«ČÜŅŗ5 mL£¬µĪ¼ÓŅ»µĪ°±Ė®ÓŠ³ĮµķÉś³É£¬ĒŅĄė×ÓÖÖĄąŌö¼Ó”£
¢ņ.ÓĆ²¬ĖæÕŗČ”ČÜŅŗ£¬ŌŚ»šŃęÉĻ×ĘÉÕ£¬Ķø¹żĄ¶É«īܲ£Į§¹Ū²ģ£¬ĪŽ×ĻÉ«»šŃę”£
¢ó.ĮķČ”ČÜŅŗ¼ÓČė¹żĮæŃĪĖį£¬ÓŠĪŽÉ«ĘųĢåÉś³É£¬øĆĪŽÉ«ĘųĢåÓöæÕĘų±ä³Éŗģ×ŲÉ«”£
¢ō.Ļņ¢óÖŠĖłµĆµÄČÜŅŗÖŠ¼ÓČėBaCl2ČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É”£
ĒėĶʶĻ£ŗ
(1)ÓÉ¢ń”¢¢ņÅŠ¶Ļ£¬ČÜŅŗÖŠŅ»¶Ø²»ŗ¬ÓŠµÄŃōĄė×ÓŹĒ______”£
( 2)¢óÖŠ¼ÓČėŃĪĖįÉś³ÉĪŽÉ«ĘųĢåµÄĄė×Ó·½³ĢŹ½ŹĒ________________”£
2)¢óÖŠ¼ÓČėŃĪĖįÉś³ÉĪŽÉ«ĘųĢåµÄĄė×Ó·½³ĢŹ½ŹĒ________________”£
(3)¼×Ķ¬Ń§×īÖÕČ·¶ØŌČÜŅŗÖŠĖłŗ¬ŃōĄė×ÓÓŠ________£¬ŅõĄė×ÓÓŠ________£»²¢¾Ż“ĖĶĘ²āŌČÜŅŗÓ¦øĆ³Ź________ŠŌ£¬ŌŅņŹĒ________________________(ĒėÓĆĄė×Ó·½³ĢŹ½ĖµĆ÷)”£
(4)ĮķČ”100 mLŌČÜŅŗ£¬¼ÓČė×ćĮæµÄNaOHČÜŅŗ£¬“Ė¹ż³ĢÖŠÉę¼°µÄĄė×Ó·½³ĢŹ½ĪŖ_____________________________________________________
___________________________________________________________ӣ
³ä·Ö·“Ó¦ŗó¹żĀĖ£¬Ļ“µÓ£¬×ĘÉÕ³ĮµķÖĮŗćÖŲ£¬µĆµ½µÄ¹ĢĢåÖŹĮæĪŖ________g”£
½āĪö””(1)øł¾Ż¢ń£¬ČÜŅŗĪŖĪŽÉ«£¬ŌņČÜŅŗÖŠ²»ŗ¬Cu2£«£¬Éś³É³ĮµķŗóĄė×ÓÖÖĄąŌö¼Ó£¬ĖµĆ÷ŌČÜŅŗÖŠ²»ŗ¬NH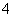 £»øł¾Ż¢ņæÉČ·¶ØČÜŅŗÖŠ²»ŗ¬K£«”£(2)øł¾Ż¢óµÄĆčŹöæÉČ·¶ØÉś³ÉµÄĪŽÉ«ĘųĢåĪŖNO£¬ŌņŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠNO
£»øł¾Ż¢ņæÉČ·¶ØČÜŅŗÖŠ²»ŗ¬K£«”£(2)øł¾Ż¢óµÄĆčŹöæÉČ·¶ØÉś³ÉµÄĪŽÉ«ĘųĢåĪŖNO£¬ŌņŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠNO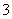 ŗĶI££¬øł¾Ż×ŖŅʵē×ÓŹżÄæĻąµČ”¢µēŗÉŹŲŗćæÉŠ“³öĄė×Ó·½³ĢŹ½”£(3)½įŗĻ¢ōŗĶČÜŅŗÖŠø÷Ąė×ÓÅضČĻąµČæÉČ·¶ØČÜŅŗÖŠŗ¬ÓŠµÄŅõĄė×ÓĪŖSO
ŗĶI££¬øł¾Ż×ŖŅʵē×ÓŹżÄæĻąµČ”¢µēŗÉŹŲŗćæÉŠ“³öĄė×Ó·½³ĢŹ½”£(3)½įŗĻ¢ōŗĶČÜŅŗÖŠø÷Ąė×ÓÅضČĻąµČæÉČ·¶ØČÜŅŗÖŠŗ¬ÓŠµÄŅõĄė×ÓĪŖSO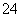 ”¢Cl£”¢NO
”¢Cl£”¢NO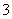 ŗĶI££¬ČÜŅŗÖŠŗ¬ÓŠµÄŃōĄė×ÓĪŖMg2£«”¢Al3£«£»½įŗĻČÜŅŗµÄ×é³ÉæÉČ·¶ØČÜŅŗĻŌĖįŠŌ£¬ŹĒMg2£«”¢Al3£«Ė®½āµÄŌµ¹Ź”£(4)¼ÓČėNaOHČÜŅŗ£¬Mg2£«×Ŗ»ÆĪŖMg(OH)2³Įµķ£¬Al3£«×īŗó×Ŗ»ÆĪŖNaAlO2£¬×īÖÕµĆµ½0.01 mol Mg(OH)2³Įµķ£¬×ĘÉÕÖĮŗćÖŲµĆµ½0.4 g MgO”£
ŗĶI££¬ČÜŅŗÖŠŗ¬ÓŠµÄŃōĄė×ÓĪŖMg2£«”¢Al3£«£»½įŗĻČÜŅŗµÄ×é³ÉæÉČ·¶ØČÜŅŗĻŌĖįŠŌ£¬ŹĒMg2£«”¢Al3£«Ė®½āµÄŌµ¹Ź”£(4)¼ÓČėNaOHČÜŅŗ£¬Mg2£«×Ŗ»ÆĪŖMg(OH)2³Įµķ£¬Al3£«×īŗó×Ŗ»ÆĪŖNaAlO2£¬×īÖÕµĆµ½0.01 mol Mg(OH)2³Įµķ£¬×ĘÉÕÖĮŗćÖŲµĆµ½0.4 g MgO”£
“š°ø””(1)K£«”¢NH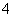 ”¢Cu2£«
”¢Cu2£«
(2)6I££«2NO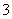 £«8H£«===3I2£«2NO”ü£«4H2O
£«8H£«===3I2£«2NO”ü£«4H2O
(3)Mg2£«”¢Al3£«””Cl£”¢NO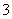 ”¢SO
”¢SO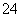 ”¢I£””Ėį””Mg2£«£«2H2OMg(OH)2£«2H£«”¢Al3£«£«3H2OAl(OH)3£«3H£«
”¢I£””Ėį””Mg2£«£«2H2OMg(OH)2£«2H£«”¢Al3£«£«3H2OAl(OH)3£«3H£«
(4)Mg2£«£«2OH£===Mg(OH)2”ż”¢Al3£«£«4OH£===AlO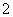 £«2H2O””0.4
£«2H2O””0.4


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻąĶ¬ĪĀ¶ČĻĀ£¬ÓŠĻĀĮŠČżøöČČ»Æѧ·½³ĢŹ½£ŗ
£Ø1£©2H2£Øl£©+O2£Øg£©=== 2H2O£Øl£© ”÷H1= -Q1 kJ• mol-1
£Ø2£©2H2£Øg£©+O2£Øg£©=== 2H2O£Øl£© ”÷H1= -Q2 kJ• mol-1
£Ø3£©2H2£Øl£©+O2£Øg£©=== 2H2O£Øg£© ”÷H1= -Q3 kJ• mol-1
ŌņQ1”¢Q2”¢Q3µÄ¹ŲĻµ±ķŹ¾ÕżČ·µÄŹĒ£Ø £©
A. Q1=Q2<Q3 B. Q2 > Q1 >Q3 C. Q3> Q2> Q1 D. Q1=Q2=Q3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓūÓĆ98%µÄÅØĮņĖį(p£½1.84g”¤cm£3 )ÅäÖĘ³ÉÅضČĪŖ0.5mol”¤L£1µÄĻ”ĮņĖį500ml”£
(1) Ń”ÓƵÄÖ÷ŅŖŅĒĘ÷ÓŠ£ŗ
¢Ł__________£¬¢Ś__________£¬¢Ū__________£¬¢Ü____________£¬¢Ż____________”£
(2) Ēė½«ĻĀĮŠø÷²Ł×÷£¬°“ÕżČ·µÄŠņŗÅĢīŌŚŗįĻßÉĻ”£
A£®ÓĆĮæĶ²ĮæČ”ÅØH2SO4 B£®·“ø“µßµ¹Ņ”ŌČ
C£®ÓĆ½ŗĶ·µĪ¹Ü¼ÓÕōĮóĖ®ÖĮæĢ¶ČĻß D£®Ļ“¾»ĖłÓĆŅĒĘ÷
E£®Ļ”ŹĶÅØH2SO4 F£®½«ČÜŅŗ×ŖČėČŻĮæĘæ
Ęä²Ł×÷ÕżČ·µÄĖ³ŠņŅĄ“ĪĪŖ__________ __________________”£
(3)¼ņŅŖ»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ŁĖłŠčÅØĮņĖįµÄĢå»żĪŖ____________mL”£
(4)×ŖŅĘ”¢Ļ“µÓ”£ŌŚ×ŖŅĘŹ±Ó¦Ź¹ÓĆ________ŅżĮ÷£¬ŠčŅŖĻ“µÓÉÕ±2”«3“ĪŹĒĪŖĮĖ______________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻÖÓŠĪåÖÖæÉČÜŠŌĪļÖŹA”¢B”¢C”¢
D”¢E£¬ĖüĆĒĖłŗ¬Ņõ”¢ŃōĄė×Ó»„²»ĻąĶ¬£¬·Ö±šŗ¬ÓŠĪåÖÖŃōĄė×ÓNa£«”¢Al3£«”¢Mg2£«”¢Ba2£«”¢Fe3£«ŗĶĪåÖÖŅõĄė×ÓCl£”¢OH£”¢NO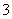 ”¢CO
”¢CO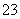 ”¢XÖŠµÄŅ»ÖÖ”£
”¢XÖŠµÄŅ»ÖÖ”£
(1)ijĶ¬Ń§Ķعż±Č½Ļ·ÖĪö£¬ČĻĪŖĪŽŠč¼ģŃé¾ĶæÉÅŠ¶ĻĘäÖŠ±ŲÓŠµÄĮ½ÖÖĪļÖŹŹĒ________ŗĶ________(Ģī»ÆѧŹ½)”£
(2)ĪŖĮĖČ·¶ØX£¬ĻÖ½«(1)ÖŠµÄĮ½ÖÖĪļÖŹ¼ĒĪŖAŗĶB£¬ŗ¬XµÄĪļÖŹ¼ĒĪŖC£¬µ±CÓėBµÄČÜŅŗ»ģŗĻŹ±£¬²śÉśŗģŗÖÉ«³ĮµķŗĶĪŽÉ«ĪŽĪ¶ĘųĢ壻µ±CÓėAµÄČÜŅŗ»ģŗĻŹ±²śÉś×Ų»ĘÉ«³Įµķ£¬ĻņøĆ³ĮµķÖŠµĪČėĻ”ĻõĖį³Įµķ²æ·ÖČܽā£¬×īŗóĮōÓŠ°×É«³Įµķ²»ŌŁČܽā”£ŌņXĪŖ________(ĢīŃ”Ļī×ÖÄø)”£
A£®SO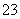 ”””””””””””””””” B£®SO
”””””””””””””””” B£®SO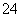
C£®CH3COO£ D£®SiO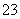
(3)½«CuĶ¶Čėµ½×°ÓŠDČÜŅŗµÄŹŌ¹ÜÖŠ£¬Cu²»Čܽā£»ŌŁµĪ¼ÓĻ”H2SO4£¬CuÖš½„Čܽā£¬¹ÜæŚø½½üÓŠŗģ×ŲÉ«ĘųĢå³öĻÖ”£ŌņĪļÖŹDŅ»¶Øŗ¬ÓŠÉĻŹöĄė×ÓÖŠµÄ________(ĢīĻąÓ¦µÄĄė×Ó·ūŗÅ)”£ÓŠ¹Ų·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_____________”£
(4)ĄūÓĆÉĻŹöŅŃ¾Č·¶ØµÄĪļÖŹ£¬æÉŅŌ¼ģŃé³öD”¢EÖŠµÄŃōĄė×Ó”£Ēė¼ņŹöŹµŃé²Ł×÷²½Öč”¢ĻÖĻó¼°½įĀŪ_______________________________________________________________________________________________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĄė×Ó·½³ĢŹ½ÖŠ£¬ÕżČ·µÄŹĒ(””””)
A£®ĻņNaOHČÜŅŗÖŠ¼ÓČėÉŁĮæMg(HCO3)2ČÜŅŗ£ŗ2OH££«Mg2£«===M g(OH)2”ż
g(OH)2”ż
B£®Ļņ×ćĮæNaHSO4ČÜŅŗÖŠÖš½„µĪČėBa(HCO3)2ČÜŅŗ£ŗHCO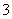 £«Ba2£«£«H£«£«SO
£«Ba2£«£«H£«£«SO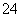 ===BaSO4”ż£«H2O£«CO2”ü
===BaSO4”ż£«H2O£«CO2”ü
C£®ĖįŠŌKMnO4ČÜŅŗÓėH2O2·“Ó¦£ŗ2MnO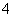 £«10H£«£«3H
£«10H£«£«3H 2O2===2Mn2£«£«3O2”ü£«8H2O
2O2===2Mn2£«£«3O2”ü£«8H2O
D£®ĻņFe(NO3)3ČÜŅŗÖŠ¼ÓČė¹żĮæHIČÜŅŗ£ŗFe3£«£«3NO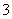 £«12H£«£«10I£===Fe2£«£«3NO”ü£«5I2£«6H2O
£«12H£«£«10I£===Fe2£«£«3NO”ü£«5I2£«6H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
H2ŗĶI2ŌŚŅ»¶ØĢõ¼žĻĀÄÜ·¢Éś·“Ó¦£ŗH2(g)£«I2(g)2HI(g)””¦¤H£½£a kJ”¤mol£1
ŅŃÖŖ£ŗ
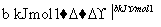

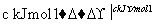
 (a”¢b”¢c¾ł“óÓŚĮć)
(a”¢b”¢c¾ł“óÓŚĮć)
ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ(””””)
A£®·“Ó¦ĪļµÄ×ÜÄÜĮæøßÓŚÉś³ÉĪļµÄ×ÜÄÜĮæ
B£®¶ĻæŖ1 mol H”ŖH¼üŗĶ1 mol I”ŖI¼üĖłŠčÄÜĮæ“óÓŚ¶ĻæŖ2 mol H”ŖI¼üĖłŠčÄÜĮæ
C£®¶ĻæŖ2 mol H”ŖI¼üĖłŠčÄÜĮæŌ¼ĪŖ(c£«b£«a) kJ
D£®ĻņĆܱÕČŻĘ÷ÖŠ¼ÓČė2 mol H2ŗĶ2 mol I2£¬³ä·Ö·“Ó¦ŗó·Å³öµÄČČĮæŠ”ÓŚ2a kJ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓĆCH4“߻ƻ¹ŌNOxæÉŅŌĻū³żµŖŃõ»ÆĪļµÄĪŪČ¾”£ŅŃÖŖCH4(g)£«4NO2(g)===4NO(g)£«CO2(g)£«2H2O(g)””¦¤H£½£574 kJ”¤mol£1£¬CH4(g)£«4NO(g)===2N2 (g)£«CO2(g)£«2H2O(g)
(g)£«CO2(g)£«2H2O(g)
¦¤H£½£1 160 kJ”¤mol£1”£ČōŌŚ±ź×¼×“æöĻĀ4.48 L CH4Ē”ŗĆÄܽ«Ņ»¶ØĮæNO2»¹Ō³ÉN2ŗĶH2O(g)£¬ŌņÕūøö¹ż³ĢÖŠ·Å³öµÄČČĮæĪŖ(””””)
A£®114.8 kJ B£®232 kJ
C£®368.8 kJ D£®173.4 kJ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĶ¼ĖłŹ¾£¬Ä³Ķ¬Ń§Éč¼ĘĮĖŅ»øöČ¼ĮĻµē³Ų²¢Ģ½¾æĀČ¼ī¹¤ŅµŌĄķŗĶ“ÖĶµÄ¾«Į¶ŌĄķ£¬ĘäÖŠŅŅ×°ÖĆÖŠXĪŖŃōĄė×Ó½»»»Ä¤”£Ēė°“ŅŖĒó»Ų “šĻą¹ŲĪŹĢā£ŗ
“šĻą¹ŲĪŹĢā£ŗ
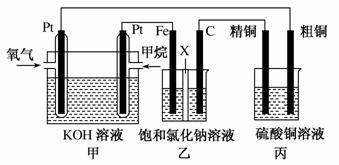
(1)¼×ĶéČ¼ĮĻµē³Ųøŗ¼«·“Ó¦Ź½ŹĒ______________________ __________________________________________________”£
(2)ŹÆÄ«(C)¼«µÄµē¼«·“Ó¦Ź½ĪŖ________________ __________________________”£
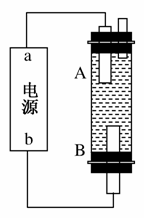
(3)ČōŌŚ±ź×¼×“æöĻĀ£¬ÓŠ2.24 LŃõĘų²Ī¼Ó·“Ó¦£¬ŌņŅŅ×°ÖĆÖŠĢś¼«ÉĻÉś³ÉµÄĘųĢåĢå»żĪŖ________L£»±ū×°ÖĆÖŠŅõ¼«Īö³öĶµÄÖŹĮæĪŖ________g”£
(4)ijĶ¬Ń§ĄūÓĆ¼×ĶéČ¼ĮĻµē³ŲÉč¼Ęµē½ā·ØÖĘČ”ĘÆ°×Ņŗ»ņFe(OH)2µÄŹµŃé×°ÖĆ(ČēĶ¼ĖłŹ¾)”£
ČōÓĆÓŚÖĘĘÆ°×Ņŗ£¬aĪŖµē³ŲµÄ____¼«£¬µē½āÖŹČÜŅŗ×īŗĆÓĆ______”£
ČōÓĆÓŚÖĘFe(OH)2£¬Ź¹ÓĆĮņĖįÄĘ×÷µē½āÖŹČÜŅŗ£¬Ńō¼«Ń”ÓĆ________×÷µē¼«”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻÖÓŠŅŅĖįŗĶĮ½ÖÖĮ“דµ„Ļ©Ģž(·Ö×ÓÖŠŗ¬Ņ»øöĢ¼Ģ¼Ė«¼ü)µÄ»ģŗĻĪļ£¬ČōĘäÖŠŃõµÄÖŹĮæ·ÖŹżĪŖa£¬ŌņĢ¼µÄÖŹĮæ·ÖŹżŹĒ(””””)
A.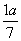 B.
B.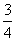 a
a
C.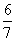 (1£a) D.
(1£a) D.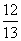 (1£a)
(1£a)
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com