
��6�֣�ij����С��ͬѧ���������ʵ��װ�ã�ͨ���ⶨijЩװ�����Լ��������仯��̽��ij��ȸʯ[��ѧʽΪaCuCO3��bCu(OH)2 ��a��bΪ������]�и�Ԫ�ص�������ϵ��
�� Ϊʹ����ȷ�����貹��װ�ã������ڷ����ڻ��װ��ͼ��д���Լ����ƣ���ע����ʯ�ҿɿ����ռ����ʯ�ҵĻ���
�� ��װ���й��������Ŀ����__����������________ ��
��װ����ҩƷ������Ϊ________ ��
ʵ��ʱ����ҩƷδ�����Ա仯��֤��____________________________________________��
���������μ��ȡ���������ȴ��������װ�õ����������������0.1g���жϸÿ�ȸʯ����ȫ�ֽ⡣
�� ����ȷ�IJⶨ�ó��������ݣ���ȸʯ���Ⱥ���ȫ�ֽ⣬������16.52 g��Ϊ12.00 g��װ��������1��44 g��д���ÿ�ȸʯ�Ļ�ѧʽ________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

 ��Ϊʹ����ȷ�����貹��װ�ã������ڷ����ڻ��װ��ͼ��д���Լ����ƣ�
��Ϊʹ����ȷ�����貹��װ�ã������ڷ����ڻ��װ��ͼ��д���Լ����ƣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪X��һ���Σ�H��һ�ֹŴ��Ͷ������������ұ�������Ľ������ʣ�F��P��J�dz����ķǽ������ʣ�I��E��G���ǹ�ҵ����Ҫ�ļ������ʣ�P��J��һ�������·�Ӧ����I����������ͼ��ʾ�Ĺ�ϵ���Իش��������⣺
��1��G�Ļ�ѧʽΪ ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
�� ��
�� ��
��3��ij����С��ͬѧ���������ʵ��װ�ã�ͨ���ⶨijЩװ�����Լ��������仯����һ��̽��X�и�Ԫ�ص�������ϵ��
�� Ϊʹ����ȷ�����貹��װ�ã������ڷ����ڻ��װ��ͼ��д���Լ����ƣ�
�� ��װ���й��������Ŀ����
____________________________________________��
��װ����ҩƷ������Ϊ________________��ʵ��ʱ����ҩƷδ�����Ա仯��֤��____________________________________________��
�� ����ж�X����ȫ�ֽ⣿
__________________________________________________
___________________________________________________________________________________________________��
�� ����ȷ�IJⶨ�ó��������ݣ�X���Ⱥ���ȫ�ֽ⣬������16��52 g��Ϊ12��00 g��װ��������1��44 g��д��X�Ļ�ѧʽ________________________��
��д��X�����ᷴӦ�Ļ�ѧ����ʽΪ�� ��
��4��L����3��Ԫ�ع��ɵķ��ӣ�����I��1��2�����ʵ���֮�ȷ�Ӧ��������
CO��NH2��2 ������M������M��ʹ�����ữ����������Һ������ɫ������д��L�ĵ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡʾ���Ը��и�����У����ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
��֪X��һ���Σ�H��һ�ֹŴ��Ͷ������������ұ�������Ľ������ʣ�F��P��J�dz����ķǽ������ʣ�I��E��G���ǹ�ҵ����Ҫ�ļ������ʣ�P��J��һ�������·�Ӧ����I����������ͼ��ʾ�Ĺ�ϵ���Իش��������⣺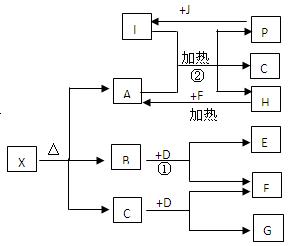
��1��G�Ļ�ѧʽΪ ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
�� ��
�� ��
��3��ij����С��ͬѧ���������ʵ��װ�ã�ͨ���ⶨijЩװ�����Լ��������仯����һ��̽��X�и�Ԫ�ص�������ϵ��
�� Ϊʹ����ȷ�����貹��װ�ã������ڷ����ڻ��װ��ͼ��д���Լ����ƣ�
�� ��װ���й��������Ŀ����
____________________________________________��
��װ����ҩƷ������Ϊ________________��ʵ��ʱ����ҩƷδ�����Ա仯��֤��____________________________________________��
�� ����ж�X����ȫ�ֽ⣿
__________________________________________________
___________________________________________________________________________________________________��
�� ����ȷ�IJⶨ�ó��������ݣ�X���Ⱥ���ȫ�ֽ⣬������16��52 g��Ϊ12��00 g��װ��������1��44 g��д��X�Ļ�ѧʽ________________________��
��д��X�����ᷴӦ�Ļ�ѧ����ʽΪ�� ��
��4��L����3��Ԫ�ع��ɵķ��ӣ�����I��1��2�����ʵ���֮�ȷ�Ӧ��������
CO��NH2��2 ������M������M��ʹ�����ữ����������Һ������ɫ������д��L�ĵ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ��Ϫ��ѧ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��6�֣�ij����С��ͬѧ���������ʵ��װ�ã�ͨ���ⶨijЩװ�����Լ��������仯��̽��ij��ȸʯ[��ѧʽΪaCuCO3��bCu(OH)2��a��bΪ������]�и�Ԫ�ص�������ϵ��
��Ϊʹ����ȷ�����貹��װ�ã������ڷ����ڻ��װ��ͼ��д���Լ����ƣ���ע����ʯ�ҿɿ����ռ����ʯ�ҵĻ���
����װ���й��������Ŀ����__����������________ ��
��װ����ҩƷ������Ϊ________ ��
ʵ��ʱ����ҩƷδ�����Ա仯��֤��____________________________________________��
���������μ��ȡ���������ȴ��������װ�õ����������������0.1 g���жϸÿ�ȸʯ����ȫ�ֽ⡣
�ܸ���ȷ�IJⶨ�ó��������ݣ���ȸʯ���Ⱥ���ȫ�ֽ⣬������16.52 g��Ϊ12.00 g��װ��������1��44 g��д���ÿ�ȸʯ�Ļ�ѧʽ________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com