
| 2 |
| 3 |
| 2 |
| 3 |
| 1mol |
| 2mol |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
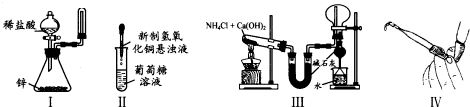
| A��ʵ��I����ȡ���ռ����� |
| B��ʵ��II����֤�����ǵĻ�ԭ�� |
| C��ʵ��III��ʵ�����ư������ռ�����İ��� |
| D��ʵ���������ʽ�ζ����Ƿ�©Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ü���ʼ��������Һ��������Һ |
B�� ʵ���Ҳ�����ͼ��ʾװ���ռ�SO2 ʵ���Ҳ�����ͼ��ʾװ���ռ�SO2 |
| C����ȥNaHCO3��Һ�е�Na2CO3���ɼ���Ca��OH��2��Һ����� |
| D������һ�����ʵ���Ũ����Һʱ������ƿ������ˮϴ�Ӻ����ô�װ��Һ��ϴ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1moL��������������ȫ��Ӧת�Ƶĵ�����ĿΪ2NA |
| B��32g������32g������O3�������е���ԭ������Ϊ2NA |
| C��100mLlmol/LFeCl3��Һ�к���Fe3+Ϊ0.1NA |
| D���ú�4molHCl��Ũ����������MnO2���ȷ�Ӧ������Cl2�ķ�����ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��23 g������������ȫȼ��ʧȥ������ΪNA |
| B����״���£�22.4L��������̼ԭ����Ϊ6NA |
| C��20�桢1.01��105Paʱ��1.6gC2H4��1.2g CO�Ļ�����壬�����������Ϊ0.1NA |
| D��0.1L 3mol?L-1��Al2��SO4��3��Һ�к��е�Al3+��ĿΪ0.6NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A |
| 3 |
| 3A |
| 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������Һ�м������������������Һ��Al3++2SO42++2Ba2++4OH-=2BaSO4��+AlO2-+2H2O |
| B����������ˮ��Cl2+H2O=2H++Cl-+ClO- |
| C����������Һ��ͨ������CO2��2C6H5O-+CO2+H2O��2C6H5OH+CO32- |
| D������������Һ��ϡ�����ϣ�Fe2++4H++NO3-=Fe3++2H2O+NO�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ�е�����ֱ�߷ֱ��ʾNa��Mg��Al��Fe��������Cl2��g����Ӧ�����Ľ�����������m���뷴Ӧ����ͬ������Cl2��g���������V��֮��Ĺ�ϵ���������δ���Na��Mg��Al��Fe��Cl2��Ӧ��ֱ���ǣ�������
��ͼ�е�����ֱ�߷ֱ��ʾNa��Mg��Al��Fe��������Cl2��g����Ӧ�����Ľ�����������m���뷴Ӧ����ͬ������Cl2��g���������V��֮��Ĺ�ϵ���������δ���Na��Mg��Al��Fe��Cl2��Ӧ��ֱ���ǣ�������| A���٢ڢۢ� | B���ܢۢڢ� |
| C���ۢڢܢ� | D���ܢڢ٢� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com