
ĻõĖįŹĒ¼«ĘäÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬¹¤ŅµÉĻ½«²śÉśµÄNO2ŌŚĆܱÕČŻĘ÷ÖŠÓĆĖ®¶ą“Ī·“ø“Ń»·ĪüŹÕÖʱøĻõĖį”£
(1)¹¤ŅµÉĻÓĆĖ®ĪüŹÕNO2Éś²śHNO3£¬Éś³ÉµÄĘųĢ徶ą“ĪŃõ»Æ”¢ĪüŹÕµÄŃ»·²Ł×÷³ä·Ö×Ŗ»ÆĪŖĻõĖį(¼Ł¶ØÉĻŹö¹ż³ĢĪŽĘäĖūĖšŹ§)”£ŹŌŠ“³öÉĻŹö·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
ӣ
(2)ĪŖĮĖÖ¤Ć÷NOŅ²æÉŅŌÓėŃõĘųŗĶĖ®¹²Ķ¬·“Ӧɜ³ÉHNO3£¬Ä³Ń§ÉśÉč¼ĘĮĖČēĶ¼ĖłŹ¾×°ÖĆ(ÓŠ¹Ų¼Š³Ö×°ÖĆŅŃĀŌČ„)”£
¢Ł¼ģ²é×°ÖĆĘųĆÜŠŌĮ¼ŗĆŗó£¬ĪŖ¹Ū²ģµ½NOĘųĢåÉś³É£¬“ņæŖK1£¬¹Ų±ÕK2£¬Ó¦“ÓUŠĪ¹ÜµÄ³¤¹ÜæŚ×¢ČėĻ”ĻõĖįÖĮ ŗó£¬ŃøĖŁ¹Ų±ÕK1£¬¹Ū²ģµ½UŠĪ¹ÜÄŚµÄĻÖĻóŹĒ ”£
¢Ś×°ÖĆ¢óÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
¢ŪÕŗNaOHČÜŅŗµÄĆŽ»ØĶŵÄ×÷ÓĆŹĒ ”£
¢Ü“ņæŖK2£¬ÖĮ×°ÖĆ¢ņÖŠ³¤²£Į§¹ÜÖŠµÄĘųĢå³ŹĪŽÉ«ŗ󣬓ņæŖK3£¬·“Ó¦Ņ»¶ĪŹ±¼äŗ󣬳¤²£Į§¹ÜÖŠ²¢Ī“³äĀśŅŗĢ唣Éč¼Ę¼ņµ„·½·Ø¼ģŃ鳤²£Į§¹ÜÖŠµÄĘųĢåŹĒ·ńŗ¬NO ”£
(1)4NO2£«O2£«2H2O=4HNO3(»ņ·Ö³ÉĮ½øö·½³ĢŹ½Š“)
(2)¢ŁUŠĪ¹ÜÓŅ²ą½ŗČūĻĀŃŲ””UŠĪ¹Ü×ó¶ĖŅŗĆęøßÓŚÓŅ¶Ė£¬ĶĖæĀżĀżČܽā£¬²śÉśĪŽÉ«ĘųĢ壬ČÜŅŗÖš½„±äĄ¶(ÖĮÉŁ“šČżµć)
¢Ś2H2O2 2H2O£«O2”ü
2H2O£«O2”ü
¢ŪĪüŹÕµŖµÄŃõ»ÆĪļ·ĄÖ¹ĪŪČ¾»·¾³
¢ÜŌŁ“ņæŖK3£¬Čō¹Ū²ģµ½³¤²£Į§¹ÜÖŠĘųĢåŃøĖŁ±äĪŖŗģ×ŲÉ«£¬ŌņÖ¤Ć÷ÓąĘųŗ¬NO£¬ČōĪŽŃÕÉ«±ä»Æ£¬ŌņÖ¤Ć÷²»ŗ¬NO(ĘäĖūŗĻĄķ“š°øŅ²æÉ)
½āĪö



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
»ÆŗĻĪļAÓÉN”¢HĮ½ÖÖŌŖĖŲ×é³É£¬0.01mol AÓė×ćĮæCuO³ä·Ö·“Ӧɜ³ÉŗģÉ«CuµÄĶ¬Ź±£¬Éś³ÉĮĖ0.36gĖ®ŗĶ±ź×¼×“æöĻĀµÄĢå»żĪŖ0.224LµÄN2”£BŹĒÓÉNaŗĶHĮ½ŌŖĖŲ×é³ÉµÄĄė×Ó»ÆŗĻĪļ£¬ÄÜÓėĖ®·“Ӧɜ³ÉH2£¬³ĘĪŖ²śĒā¼Į”£»ÆŗĻĪļCµÄŃęÉ«·“Ó¦ĪŖ»ĘÉ«£¬øł¾ŻÖŹĘ×·ÖĪöĖüµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ39ĒŅÓėĖ®·“Ó¦ÄÜÉś³É°±Ęų”£
£Ø1£©AµÄ»ÆѧŹ½ĪŖ £»AÓėCuO·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©BµÄµē×ÓŹ½ĪŖ ”£
£Ø3£©CÖŠ“ęŌŚµÄ»Æѧ¼ü £»CÓė¹żĮæĻ”ŃĪĖį·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©ÓŠŅ»ÖÖĄķĻėµÄ»ÆŗĻĪļNH4H£¬¾²éŌÄ׏ĮĻ£¬øĆĪļÖŹÖĮ½ńĪ“ÄÜÖĘµĆ£¬ĒėĖµĆ÷æÉÄܵÄŌŅņ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijŗ¬ĀČ»ÆŗĻĪļAÓÉĮ½ÖÖ¶ĢÖÜĘŚŌŖĖŲ×é³É£¬³£ĪĀĻĀøĆĪļÖŹĪŖĘųĢ¬£¬²āµĆøĆĘųĢå¶ŌæÕĘųµÄĻą¶ŌĆܶČĪŖ3.0£¬AČÜÓŚĖ®æɵĆÖ»ŗ¬µ„Ņ»ČÜÖŹBµÄČõĖįŠŌČÜŅŗ£¬BČÜŅŗŌŚ·ÅÖĆ¹ż³ĢÖŠĘäĖįŠŌ»įŌöĒ攣³£ĪĀĻĀ£¬ĘųĢåAÓėNH3·“Ӧɜ³ÉĄė×Ó¾§ĢåC”¢ĘųĢåµ„ÖŹDŗĶ³£¼ūŅŗĢåE£¬DĪŖæÕĘųÖŠŗ¬Įæ×ī¶ąµÄĪļÖŹ”£ĘųĢåAæÉÓĆijŅ»ĘųĢåµ„ÖŹÓė³±ŹŖµÄNa2CO3·“Ó¦ÖĘµĆ£¬Ķ¬Ź±Éś³ÉĮ½ÖÖÄĘŃĪ”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĘųĢåAµÄ»ÆѧŹ½ĪŖ £¬ĘųĢåµ„ÖŹD¶ŌÓ¦ŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆĪŖ ”£
£Ø2£©ÓĆ»Æѧ·½³ĢŹ½±ķŹ¾BČÜŅŗĖįŠŌŌöĒæµÄŌŅņ ”£
£Ø3£©ĘųĢåAÓėNH3·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £¬øĆ·“Ó¦ĢåĻÖĘųĢåA¾ßÓŠ ŠŌ”£
£Ø4£©ŹŌŠ“³öÖĘČ”ĘųĢåAµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø5£©Éč¼ĘŹµŃéĢ½¾æĄė×Ó¾§ĢåCµÄ³É·ÖĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijŠ£»ÆѧŹµŃ銔×éĪŖĮĖÖ¤Ć÷ĶÓėĻ”ĻõĖį·“Ó¦²śÉśŅ»Ńõ»ÆµŖ£¬ÓĆČēĶ¼ĖłŹ¾×°ÖĆ½ųŠŠŹµŃé(¼ÓČČ×°ÖĆŗĶ¼Š³Ö×°ÖĆ¾łŅŃĀŌČ„£¬×°ÖĆĘųĆÜŠŌĮ¼ŗĆ£¬FŹĒÓĆÓŚ¹ÄČėæÕĘųµÄĖ«Į¬“ņĘųĒņ)”£
ŹµŃé²Ł×÷¼°ĻÖĻó£ŗ
| ŹµŃé²Ł×÷ | ĻÖĻó |
| ¢ń.½«B×°ÖĆĻĀŅĘ£¬Ź¹Ģ¼ĖįøĘÓėĻ”ĻõĖį½Ó“„ | ²śÉśĘųĢå |
| ¢ņ.µ±C×°ÖĆÖŠ²śÉś°×É«³ĮµķŹ±£¬Į¢æĢ½«B×°ÖĆÉĻĢį | |
| ¢ó.½«A×°ÖĆÖŠĶĖæ·ÅČėĻ”ĻõĖįÖŠ£¬øųA×°ÖĆĪ¢Ī¢¼ÓČČ | A×°ÖĆÖŠ²śÉśĪŽÉ«ĘųĢå E×°ÖĆÖŠæŖŹ¼Ź±³öĻÖĒ³ŗģ×ŲÉ«ĘųĢå |
| ¢ō.ÓĆF×°ÖĆĻņE×°ÖĆÖŠ¹ÄČėæÕĘų | E×°ÖĆÄŚĘųĢåŃÕÉ«Öš½„¼ÓÉī |
| ¢õ.Ņ»¶ĪŹ±¼äŗó | C×°ÖĆÖŠ°×É«³ĮµķČܽā |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĀČĘųŹĒŅ»ÖÖ·Ē³£ÖŲŅŖµÄ»Æ¹¤ŌĮĻ£¬ŗ¬ĀČĻū¶¾¼ĮŌŚÉś²śÉś»ī֊ӊ׏ć·ŗµÄÓĆĶ¾”£
(1)ÓĆĀČĘųæÉÖĘČ””°84Ļū¶¾Ņŗ”±(ÓŠŠ§³É·ÖĪŖNaClO)”£
¢ŁøĆÖʱø·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
¢ŚĻĀĮŠ“ėŹ©ÄÜŌöĒæ”°84Ļū¶¾Ņŗ”±É±¾śÄÜĮ¦µÄŹĒ ”£
A£®¼ÓČėŹŹĮæ“×Ėį
B£®¼ÓČėŹŹĮæŃĒĮņĖį
C£®¼ÓČėÉŁĮæNaOH·ŪÄ©
¢Ū”°84Ļū¶¾Ņŗ”±²»ÄܶŌøÖĢś(ŗ¬Fe”¢C)ÖĘĘ·½ųŠŠĻū¶¾£¬ŌŅņŹĒ ”£
(2)¶žŃõ»ÆĀČ(ClO2)ŹĒŅ»ÖÖ»ĘĀĢÉ«ĘųĢ壬ŹĒ¹ś¼ŹÉĻ¹«ČĻµÄøߊ§”¢¹ćĘ×”¢æģĖŁ”¢°²Č«µÄɱ¾śĻū¶¾¼Į”£ÄæĒ°£¬¹¤ŅµÉĻæŖ·¢³öÓƵē½ā·ØÖĘČ”ClO2µÄŠĀ¹¤ŅÕ£¬Éś²śŌĄķČēĶ¼ĖłŹ¾”£
ÉĻŹöŹ¾ŅāĶ¼ÖŠÓĆŹÆÄ«×÷µē¼«£¬ŌŚŅ»¶ØĢõ¼žĻĀµē½ā±„ŗĶŹ³ŃĪĖ®ÖĘČ”ClO2”£Š“³öŃō¼«²śÉśClO2µÄµē¼«·“Ó¦Ź½£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijĶ¬Ń§¶ŌCl2ÓėKIČÜŅŗµÄ·“Ó¦½ųŠŠĮĖŹµŃéĢ½¾æ”£·“ӦװÖĆČēĻĀ£ŗ
ĶØČėĀČĘųŅ»¶ĪŹ±¼ä£¬KIČÜŅŗ±äĪŖ»ĘÉ«”£¼ĢŠųĶØČėĀČĘųŅ»¶ĪŹ±¼äŗó£¬ČÜŅŗ»ĘÉ«ĶŹČ„£¬±äĪŖĪŽÉ«”£¼ĢŠųĶØČėĀČĘų£¬×īŗóČÜŅŗ±äĪŖĒ³»ĘĀĢÉ«”£
£Ø1£©ŅŃÖŖI2£«I£ I3£ £¬I2”¢I3£ŌŚĖ®ÖŠ¾ł³Ź»ĘÉ«”£ĪŖČ·¶Ø»ĘÉ«ČÜŅŗµÄ³É·Ö£¬½ųŠŠĮĖŅŌĻĀŹµŃ锣
I3£ £¬I2”¢I3£ŌŚĖ®ÖŠ¾ł³Ź»ĘÉ«”£ĪŖČ·¶Ø»ĘÉ«ČÜŅŗµÄ³É·Ö£¬½ųŠŠĮĖŅŌĻĀŹµŃ锣
| | ²Ł×÷ | ŹµŃéĻÖĻó |
| a | Č”2~3 mL»ĘÉ«ČÜŅŗ£¬¼ÓČė×ćĮæCCl4£¬ Õńµ“¾²ÖĆ”£ | CCl4²ć³Ź×ĻŗģÉ«£¬ Ė®²ćĻŌĒ³»ĘÉ«”£ |
| b | Č”2~3 mL±„ŗĶµāĖ®£¬¼ÓČė×ćĮæCCl4£¬ Õńµ“¾²ÖĆ”£ | CCl4²ć³Ź×ĻŗģÉ«£¬Ė®²ć¼ø½üĪŽÉ«”£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ä³ŃŠ¾æŠŌѧĻ°Š”×éÉč¼ĘĮ½Ģ׏µŃé·½°øÖʱø°±Ęų²¢Ģ½¾æĘ仹ŌŠŌ(²æ·Ö¼Š³Ö×°ÖĆŅŃŹ”ĀŌ)”£
¼×·½°ø£ŗČēĶ¼ĖłŹ¾”£
ŅŅ·½°ø£ŗČēĶ¼ĖłŹ¾”£
øł¾Ż·½°ø»Ų“šĻĀĮŠĪŹĢā£ŗ
(Ņ»)¼×·½°ø
(1)B×°ÖĆŹ¢×°¼īŹÆ»Ņ£¬A×°ÖĆÖŠŅ©Ę·æÉŅŌŹĒ ”£
a£®ĀČ»Æļ§¹ĢĢåŗĶÉÕ¼ī b£®Ģ¼ĖįĒāļ§¹ĢĢå
c£®ĀČ»Æļ§¹ĢĢå d£®ĮņĖįļ§ŗĶŹģŹÆ»Ņ
(2)ŹµŃéĶź±Ļŗó£¬Éč¼Ę¼ņµ„ŹµŃé¼ģŃéDŹŌ¹ÜŹÕ¼Æµ½µÄĪļÖŹ(¼ņŹö²Ł×÷¹ż³Ģ”¢ĻÖĻóŗĶ½įĀŪ) ”£
(3)Š“³öCÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
(¶ž)ŅŅ·½°ø
(4)AĪŖÅØ°±Ė®£¬BĪŖÉśŹÆ»Ņ£¬¼ņŹöŹµŃéŌĄķ£ŗ ”£E×°ÖĆĄļŹ¢×°µÄŅ©Ę·ŹĒ ”£
(5)ÄÜÖ¤Ć÷°±Ęų¾ßÓŠ»¹ŌŠŌµÄŹµŃéĻÖĻó ”£(ČĪŠ“Į½Ģõ)
(6)ĶÓŠ£«2¼Ū”¢£«1¼Ū”¢0¼Ū£¬øł¾ŻŃõ»Æ»¹Ō·“Ó¦ŌĄķ£¬D²£Į§¹ÜĄļ»¹Ō²śĪļ³żĶĶā£¬»¹æÉÄÜÓŠ £¬¼ŁÉčÓŠĶŅŌĶāµÄ»¹Ō²śĪļÉś³É£¬Š“³öDÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijŹŌ¼Į³§ÓĆŅų£Øŗ¬ŌÓÖŹĶ£©ŗĶĻõĖį£Øŗ¬ŌÓÖŹFe3+£©·“Ó¦ÖĘČ”ĻõĖįŅų”£²½ÖčČēĻĀ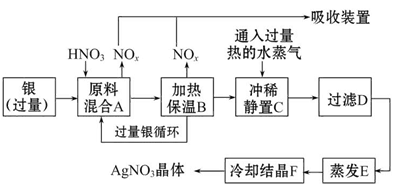
ŅĄ¾ŻÉĻŹö²½Öč£¬Ķź³ÉĻĀĮŠĢīæÕ£ŗ
£Ø1£©ČܽāŅųµÄĻõĖįÓ¦øĆÓĆ________ĻõĖį£ØĢī”°ÅØ”±»ņ”°Ļ””±£©”£ŌŅņŹĒ________ £ØĢīŠņŗÅ£¬ĻĀĶ¬£©
a.¼õÉŁ¹ż³ĢÖŠ²śÉśNOxµÄĮæ
b.¼õÉŁŌĮĻŅųµÄĻūŗÄĮæ
c.½ŚŹ”ĻõĖįµÄĪļÖŹµÄĮæ
£Ø2£©²½ÖčB¼ÓČȱ£ĪĀµÄ×÷ÓĆŹĒ________”£
a.ÓŠĄūÓŚ¼Óæģ·“Ó¦ĖŁĀŹ
b.ÓŠĄūÓŚĪ“·“Ó¦µÄĻõĖį»Ó·¢
c.ÓŠĄūÓŚĻõĖį³ä·Ö·“Ó¦£¬½µµĶČÜŅŗÖŠc£ØH+£©
£Ø3£©²½ÖčCÖŠŹĒĪŖĮĖ³żČ„Fe3+”¢Cu2+µČŌÓÖŹ£¬³åĻ”¾²ÖĆŹ±·¢ÉśµÄ»Æѧ·“Ó¦ŹĒ________”£
a.ÖĆ»»·“Ó¦
b.Ė®½ā·“Ó¦
c.Ńõ»Æ»¹Ō·“Ó¦
²śÉśµÄ³ĮµķĪļ»ÆѧŹ½________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¶žŃõ»ÆĮņŹĒĮņµÄÖŲŅŖ»ÆŗĻĪļ£¬ŌŚÉś²ś”¢Éś»īÖŠÓŠ¹ć·ŗÓ¦ÓĆ”£¶žŃõ»ÆĮņÓŠ¶¾£¬²¢ĒŅŹĒŠĪ³ÉĖįÓźµÄÖ÷ŅŖĘųĢ唣ĪŽĀŪŹĒŹµŃéŹŅÖʱø»¹ŹĒ¹¤ŅµÉś²ś£¬¶žŃõ»ÆĮņĪ²ĘųĪüŹÕ»ņŃĢĘųĶŃĮņ¶¼·Ē³£ÖŲŅŖ”£Ķź³ÉĻĀĮŠĢīæÕ£ŗ
(1)ŹµŃéŹŅæÉÓĆĶŗĶÅØĮņĖį¼ÓČČ»ņĮņĖįŗĶŃĒĮņĖįÄĘ·“Ó¦ÖĘČ”¶žŃõ»ÆĮņ”£
Čē¹ūÓĆĮņĖįŗĶŃĒĮņĖįÄĘ·“Ó¦ÖĘČ”¶žŃõ»ÆĮņ£¬²¢Ļ£ĶūÄÜæŲÖĘ·“Ó¦ĖŁ¶Č£¬ÉĻĶ¼ÖŠæÉŃ”ÓƵķ¢Éś×°ÖĆŹĒ (ĢīŠ“×ÖÄø)”£
(2)ČōÓĆĮņĖįŗĶŃĒĮņĖįÄĘ·“Ó¦ÖĘČ”3.36L(±ź×¼×“æö)¶žŃõ»ÆĮņ£¬ÖĮÉŁŠčŅŖ³ĘČ”ŃĒĮņĖįÄĘ g(±£ĮōŅ»Ī»Š”Źż)£»Čē¹ūŅŃÓŠ4.0%ŃĒĮņĖįÄĘ(ÖŹĮæ·ÖŹż)£¬±»Ńõ»Æ³ÉĮņĖįÄĘ£¬ŌņÖĮÉŁŠč³ĘČ”øĆŃĒĮņĖįÄĘ g(±£ĮōŅ»Ī»Š”Źż)”£
(3)ŹµŃéŹŅ¶žŃõ»ÆĮņĪ²ĘųĪüŹÕÓė¹¤ŅµŃĢĘųĶŃĮņµÄ»ÆѧŌĄķĻąĶØ”£
ŹÆ»Ņ”ŖŹÆøą·ØŗĶ¼ī·ØŹĒ³£ÓƵÄŃĢĘųĶŃĮņ·Ø”£ŹÆ»Ņ-ŹÆøą·ØµÄĪüŹÕ·“Ó¦ĪŖ£ŗSO2+Ca(OH)2=CaSO3”ż+H2O”£ĪüŹÕ²śĪļŃĒĮņĖįøĘÓɹܵĄŹäĖĶÖĮŃõ»ÆĖžŃõ»Æ£¬·“Ó¦ĪŖ2CaSO3+O2+4H2O=2CaSO4”¤2H2O”£ĘäĮ÷³ĢČēĻĀĶ¼£ŗ

¼ī·ØµÄĪüŹÕ·“Ó¦ĪŖSO2+2NaOH=Na2SO3+H2O”£¼ī·ØµÄĢŲµćŹĒĒāŃõ»ÆÄĘ¼īŠŌĒ攢ĪüŹÕæģ”¢Š§ĀŹøß”£ĘäĮ÷³ĢČēĻĀĶ¼£ŗ
ŅŃÖŖ£ŗ
| ŹŌ¼Į | Ca(OH)2 | NaOH |
| ¼Ūøń(ŌŖ/kg) | 0.36 | 2.9 |
| ĪüŹÕSO2µÄ³É±¾(ŌŖ/mol) | 0.027 | 0.232 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com