
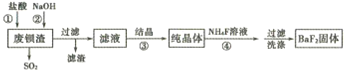
����Ŀ�����÷ϱ���(��Ҫ�ɷ�ΪBaS2O3��������SiO2)Ϊԭ�������ߴ�������������������
��֪:Kap(BaS2O3)=6.96��10-11��Kap(BaF2)=1.0��10-6
(1)����ٳ�����SO2�������е���ɫ�������ɣ��÷�Ӧ�����ӷ���ʽΪ______________��
(2)����ڵ�Ŀ�����к���������������NaOH��Һ���˹�������ԭ����__________(�����ӷ���ʽ��ʾ)��
(3)��Һ����Ҫ�ɷ���BaCl2������������NaCl���ܽ���������±���
�¶� | 20�� | 40�� | 60�� | 80�� | 100�� |
NaCl | 36.0g | 36.6g | 37.3g | 39.0g | 39.8g |
BaCl2 | 35.8g | 40.8g | 46.2g | 52.5g | 59.4g |
������˲���_____ (���������ᾧ���������½ᾧ��)��
(4)��ҵ�Ͽ��ð�ˮ����SO2����ͨ�����ʹ��ת��Ϊ�̬���ʡ���ת�����������뻹ԭ�������ʵ���֮��Ϊ__________��
(5)���������BaF2�ķ�Ӧ����Ϊ____________��
�����÷�Ӧ�¶ȹ��ߣ��������c(F-)���͵�ԭ����__________��
���о��������ʵ�����NH4F�ı������������BaF2�IJ��ʺʹ��ȣ���Ũ��Ϊ0. 1mol��L-1��BaCl2��Һ��0.22 mol��L-1NH4F��Һ����������������Һ��c(Ba2+)=__________ mol��L-1��
���𰸡� BaS2O3+2H+=Ba2++S��+SO2��+H2O 2OH-+SiO2=SiO32-+H2O ���½ᾧ 1:2 ���ֽⷴӦ �¶Ƚϸߴٽ�F-ˮ��,ʹc(F-)���� 0.01
��������������(1)��������ͼ��������зϱ����е�BaS2O3�����ᷴӦ����SO2������
(2)����ڵ�Ŀ�����к��������ᣬ�������������ƻ���������跴Ӧ��
(3)����BaCl2��NaCl�ܽ�����ݿ�֪���Ȼ������ܽ�����¶ȵı仯���Ȼ��Ƶ��ܽ�����¶ȵı仯��ͬ��
(4)ת����������Ϊ��������ԭ��ΪSO32-��SO2�����ݵ�ʧ�����غ������
(5)���������BaF2�Ļ�ѧ��Ӧ����ʽΪBaCl2+2NH4F=BaF2+2NH4Cl���������ˮ�����¶ȵ�Ӱ�죬�¶�Խ�ߣ�ˮ��̶�Խ�����ڸ���BaCl2+2NH4F=BaF2+2NH4Cl���㷴Ӧ����Һ��c(F-)���ٸ���Kap(BaF2)����c(Ba2+)��
��⣺(1)������зϱ���(��Ҫ�ɷ�ΪBaS2O3��������SiO2)�е�BaS2O3�����ᷴӦ������SO2�⣬���е���ɫ���������ɣ���Ӧ�����ӷ���ʽΪBaS2O3+2H+=Ba2++S��+SO2��+H2O���ʴ�Ϊ��BaS2O3+2H+=Ba2++S��+SO2��+H2O��
(2)����ڵ�Ŀ�����к��������ᣬ����NaOH��Һ���˹������������������ƻ���������跴Ӧ�����ӷ���ʽΪ2OH-+SiO2=SiO32-+H2O���ʴ�Ϊ��2OH-+SiO2=SiO32-+H2O��
(3)��Һ����Ҫ�ɷ���BaCl2������������NaCl�����ݶ����ܽ�����ݿ�֪���Ȼ������ܽ�����¶ȵı仯�Ƚ����ԣ����Ȼ��Ƶ��ܽ�����¶ȵı仯��������˲��ý��½ᾧ���ʴ�Ϊ�����½ᾧ��
(4)��ҵ�Ͽ��ð�ˮ����SO2����ͨ�����ʹ��ת��Ϊ�̬���ʣ���ת����������Ϊ��������ԭ��ΪSO32-��SO2�����ݵ�ʧ�����غ㣬�������ʵ���֮��Ϊ1:2���ʴ�Ϊ��1:2��
(5)���������BaF2�Ļ�ѧ��Ӧ����ʽΪBaCl2+2NH4F=BaF2+2NH4Cl�����ڸ��ֽⷴӦ���ʴ�Ϊ�����ֽⷴӦ��
�����÷�Ӧ�¶ȹ��ߣ��¶Ƚϸߴٽ�F-ˮ�⣬ʹc(F-)���ͣ��ʴ�Ϊ���¶Ƚϸߴٽ�F-ˮ�⣬ʹc(F-)������
��������Һ��c(F-)=![]() ��(0.22 mol��L-1-0.2 mol��L-1)=0.01 mol��L-1��c(Ba2+)=
��(0.22 mol��L-1-0.2 mol��L-1)=0.01 mol��L-1��c(Ba2+)=![]() =
=![]() = mol��L-1���ʴ�Ϊ��0.01��
= mol��L-1���ʴ�Ϊ��0.01��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾװ�ôӺ�ˮ����ȡCO2,�����ڼ��ٻ�������������ĺ���������˵����ȷ����

A. ͨ���a�ҵ�pH����
B. �м���ҷ����ķ�Ӧ:HCO3-+H+==CO2��+H2O���Ӷ���ȡCO2
C. ������ӦʽΪ2H+-2e-==H2��
D. ����·��ͨ��1mol���ӵĵ���ʱ������22.4LCO2�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��ͬ��ѧԪ������ɵĻ��������˵������ȷ����

A. ��ͼ�Т�Ϊij�ֶ����ĵ��壬���������ǰ�����
B. ���ڴ�����Ƥ�º�����������Χ�Ȳ�λ�������֬��
C. ��һ���Ǻ��������ɺ���ĵ��������
D. �����Dz��빹��ֲ��ϸ���ڵ�һ�ֶ��ǣ�������������ά��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������и������ʰ����ʡ��ᡢ��η���˳�����У�������ȷ���ǣ� ��
A.ˮ�������ᡢ�ռ��������B.��ˮ�����ᡢ�����ᱵ
C.���������ᡢ�������D.ͭ�����ᡢʯ��ʯ���Ȼ�ͭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�²�ϸ�����ڵ��ⶾ�أ���ɫ��ϸ��״�ᾧ����С��������к�ǿ�Ķ��ԣ����������ѡ�Ż�¡���Ѫ�����εȣ��������������ⶾ��Ϊ��״�ģ��ṹʽ��ͼ��ʾ�����ͼ�����ش�

��1���û������к�������İ���_____________�����Ȼ�________________����
��2���û���������_____________����������ɵģ�������Щ�������������������ṹ�е�_____________��
��3����ɸû�����İ�������___________�֣�������_____________���������R����ͬ�����R����_______________��
��4���û������Ϊ��״__________�Ļ��������___________���ļ���
��5����д���߿��ڽṹ�����ƣ�A._________________��B.___________________��
��6���û��������8����ԭ�ӣ�����_____________��λ���ļ��ϣ�____________��λ��R���ϡ�
��7�����ⶾ�ػ������γɹ�����ʧȥ��______________��ˮ���ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�²�ϸ�����ڵ��ⶾ�أ���ɫ��ϸ��״�ᾧ����С��������к�ǿ�Ķ��ԣ����������ѡ�Ż�¡���Ѫ�����εȣ��������������ⶾ��Ϊ��״�ģ��ṹʽ��ͼ��ʾ�����ͼ�����ش�

��1���û������к�������İ���_____________�����Ȼ�________________����
��2���û���������_____________����������ɵģ�������Щ�������������������ṹ�е�_____________��
��3����ɸû�����İ�������___________�֣�������_____________���������R����ͬ�����R����_______________��
��4���û������Ϊ��״__________�Ļ��������___________���ļ���
��5����д���߿��ڽṹ�����ƣ�A._________________��B.___________________��
��6���û��������8����ԭ�ӣ�����_____________��λ���ļ��ϣ�____________��λ��R���ϡ�
��7�����ⶾ�ػ������γɹ�����ʧȥ��______________��ˮ���ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ϊ��ȡ�л���Z�ķ�Ӧ������˵����ȷ����
![]()
A. X��Y���ܷ���ȡ����Ӧ��Z���ܷ��� B. X��Y��Z��ֻ��X������ԭ�ӿ��ܹ���
C. X��Y������Ϊ�ϳɾۺ���ĵ��� D. Y��ͬ���칹�����Ŀֻ��2��(����������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Һ�����ܽ�Al(OH)3�������ܽ�Al���������ܽ�Fe���ڸ���Һ�п��Դ���������������� (����)
A. K����Na����HCO3����NO3�� B. Na����SO42����Cl����S2��
C. NH4����Mg2����SO42����NO3�� D. H����K����Cl����Na��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ȷ���������������ƹ�������0.20 mol��L��1������������Һ250 mL��Ҫ�õ���������(����)
��250 mL����ƿ����������ƽ������ƿ���ܽ�ͷ�ιܡ����ձ��������������Թܡ���ҩ��
A. �٢ܢݢ� B. �٢ڢܢ�
C. �٢ڢܢݢޢ� D. ȫ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com