

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
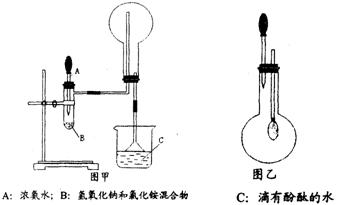
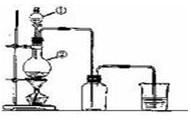
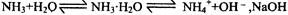 ʹƽ�������ƶ�
ʹƽ�������ƶ�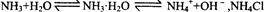 ʹƽ�������ƶ�
ʹƽ�������ƶ�
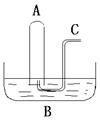
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
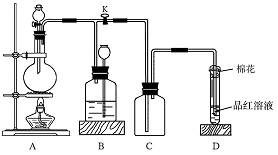

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
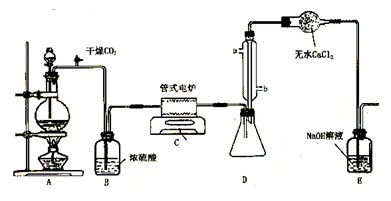
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��û�����ܡ��ѵ��롢�ӷ��������ɵĸ��ֽⷴӦҲ���ܷ��� |
| B����Һ��ijЩ���ӽ�ϳ������������������ԭ������Щ����Ũ�ȵ��ݴλ������˸����ʵ��ܶȻ����������ƽ�ⳣ�� |
| C��һ���������ʻ��ѵ������ʣ�����ͨ�����ֽⷴӦ���ɸ����ܻ���ѵ�������� |
| D��ֻҪ�ʵ��ı��¶ȡ�ѹǿ��Ũ�ȣ��κη�Ӧ�����Է����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| ѡ�� | ��ȡ���� | a | b | c | d |
| A | NO | ϡ���� | ͭƬ | H2O | Ũ���� |
| B | O2 | ˫��ˮ | MnO2 | H2O | Ũ���� |
| C | NH3 | Ũ��ˮ | ��ʯ�� | Ũ���� | H2O |
| D | SO2 | Ũ���� | Na2SO3��ĩ | NaHSO3��Һ | Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Խ�̿��ˮ��ȡˮú������������ |
| B��������п�Ȼ��ý�����ϡ���ᷴӦ��ȡ���� |
| C���ɻ������糧�ṩ�������ˮ�������� |
| D�����ø�Ч������̫����ʹ��ˮ�ֽ�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com