
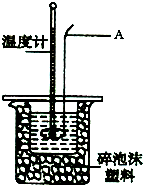
 ijʵ��С����0.50mol?L-1NaOH��Һ��0.50mol?L-1��1������Һ�����к��ȵIJⶨ��
ijʵ��С����0.50mol?L-1NaOH��Һ��0.50mol?L-1��1������Һ�����к��ȵIJⶨ��| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �¶Ȳt2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.6 | 26.6 | 26.6 | 29.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 31.2 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 3.5+4+3.9+4.1 |
| 4 |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ�鲽�裨��Ҫ��д����������̣� | Ԥ������ͽ��� |
| �� �� �� | ��������ʧ������2������ ����������ʧ������2�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | K+��Ag+��Mg2+��Ba2+ |
| ������ | NO3-��CO32-��SiO32-��SO42- |
| ��� | ʵ������ | ʵ���� |
| �� | �����Һ�� ��������ϡ���� | ������ɫ�������ų���״����0.56 L���� |
| �� | ����ķ�Ӧ���Һ���ˣ��Գ���ϴ�ӡ����������أ��������ù������� | ��������Ϊ2.4 g |
| �� | ������Һ�еμ�BaCl2��Һ | ���������� |
| ������ | NO3- | CO32-�� | SiO32- | SO42- |
| c/mol?L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��0.01mol��AlCl3��Һ�еμ�2mol/L��NaOH��Һʱ�����õij������������NaOH��Һ�����֮��Ĺ�ϵ����ͼ��ʾ��
��0.01mol��AlCl3��Һ�еμ�2mol/L��NaOH��Һʱ�����õij������������NaOH��Һ�����֮��Ĺ�ϵ����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Fe��OH��2 |
| B��Fe��OH��3 |
| C��K3Fe��CN��6 |
| D��Cu��NH3��4SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��NH4Cl����NH4��2SO4��CH3COONa |
| B����NH4��2SO4��NH4Cl��CH3COONa |
| C����NH4��2SO4��NH4Cl��NaOH |
| D��CH3COOH��NH4Cl����NH4��2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
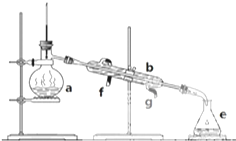 �����������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�����ͼΪʵ��װ�ã�
�����������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�����ͼΪʵ��װ�ã��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com