
��������Ҫ�ɷ�ΪFeS2���ǹ�ҵ��ȡ�������Ҫԭ�ϣ������ղ���ΪSO2��Fe3O4��
��1����0.050molSO2(g)��0.030molO2(g)�����ݻ�Ϊ1L���ܱ������У���Ӧ��
2SO2(g) + O2(g) ![]()
![]() 2SO3(g)��һ�������´ﵽƽ�⣬���c(SO3)=0.040mol?L������������·�Ӧ��ƽ�ⳣ��K��SO2��ƽ��ת���ʣ�д��������̣���
2SO3(g)��һ�������´ﵽƽ�⣬���c(SO3)=0.040mol?L������������·�Ӧ��ƽ�ⳣ��K��SO2��ƽ��ת���ʣ�д��������̣���
��2����֪������Ӧ�Ƿ��ȷ�Ӧ�����÷�Ӧ����ƽ��״̬ʱ�����������������£����д�ʩ�����������SO2ƽ��ת���ʵ��� ������ĸ��
A�������¶� B�������¶� C������ѹǿ
D����Сѹǿ E��������� G���Ƴ�����
��3��SO2β���ñ���Na2SO3��Һ���տɵõ���Ҫ�Ļ���ԭ�ϣ���Ӧ�Ļ�ѧ����ʽΪ
��
��4��������������ղ���Fe3O4����H2SO4�������ۣ����Ʊ�FeSO4�����ܹ������豣����Һ�㹻���ԣ���ԭ���� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2007����ͨ�ߵ�ѧУ����ȫ��ͳһ���ԡ���ѧ(�㶫��) ���ͣ�038
| |||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��0.050 mol SO2(g)��0.030 mol O2(g)�����ݻ�Ϊ![]() 2SO3(g)��һ�������´ﵽƽ�⣬���c(SO3)=0.040 mol��L-1�������������SO2��ƽ��ת����(д���������)��
2SO3(g)��һ�������´ﵽƽ�⣬���c(SO3)=0.040 mol��L-1�������������SO2��ƽ��ת����(д���������)��
(2)��֪������Ӧ�Ƿ��ȷ�Ӧ�����÷�Ӧ����ƽ��״̬ʱ�����������������£����д�ʩ�����������SO2ƽ��ת���ʵ���___________(����ĸ)��
A.�����¶� B.�����¶� C.����ѹǿ D.��Сѹǿ E.������� F.�Ƴ�����
(3)SO2β���ñ���Na2SO3��Һ���տɵõ�����Ҫ�Ļ���ԭ�ϣ���Ӧ�Ļ�ѧ����ʽΪ___________________________��
(4)������������ղ���Fe3O4����H2SO4�������ۣ����Ʊ�FeSO4�����ܹ������豣����Һ�㹻���ԣ���ԭ����_______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ���Ĵ�ʡüɽ�и�����һ������Կ��Ի�ѧ�Ծ��������棩 ���ͣ������
���������û�������������������ų��ķ�������Ҫ��ѧ�ɷ�ΪSiO2Լ45%��Fe2O3Լ40%��Al2O3Լ10%��MgOԼ5%��Ŀǰ�ҹ��Ѿ��ڼ�����ȡ��ͻ�ơ������������з�������ֳɷֲ��������á������̺�����������£�
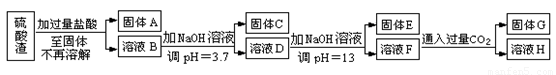
�����ϵ�֪��
|
�������� |
�ܶȻ�(Ksp) |
pHֵ |
|
|
��ʼ���� |
��ȫ���� |
||
|
Mg(OH)2 |
5.6��10��12 |
9.3 |
10.8 |
|
Fe(OH)3 |
2.8��10��16 |
2.7 |
3.7 |
|
Al(OH)3 |
1.3��10��33 |
3.7 |
4.7 |
��ش��������⣺
��1��д������A�Ļ�ѧʽΪ ��
��2����Ҫ�ⶨ��Һ��pH�Ƿ�ﵽ3.7������ʵ����Ʒ�п�ѡ�õ��� ��
A��ʯ����Һ B���㷺pH��ֽ C������pH��ֽ D��pH��
��3������������ӷ�Ӧ����ʽ
����ҺD���ɹ���E �� ����ҺF���ɹ���G ��
��4��Ҫ������C������E����G��ת��Ϊ��Ӧ���ȶ����������е�ʵ�����Ϊ ��
��5������������Һ����ı仯���������ҺH��c��Mg2����= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ��ҵ�ڹ�����ռ�м�����Ҫ�ĵ�λ������ʡ�ǹ��������������������֮һ����Ҫ����ʽ����Ҫ�豸: �����������Ҫ�ɷ�ΪFeS2�� 4FeS2+11O2 = 2Fe2O3+8SO2 ������¯�� 2SO2+O2![]() 2SO3 ���Ӵ��ң� SO3+H2O=H2SO4����������
2SO3 ���Ӵ��ң� SO3+H2O=H2SO4����������
��1�����������У����ݻ�ѧƽ��ԭ����ȷ�����������ʩ�� ����д��ţ���
A����ʯ�������¯֮ǰ�ȷ��� B���Ӵ��ҵķ�Ӧʹ��V2O5������
C���Ӵ����в�ʹ�úܸߵ��¶� D��������¯����Ҫ�й����Ŀ���
E���Ӵ����е������ڳ�ѹ�½��� F������������98.3%��Ũ��������SO3
��2��0.1mol/L��NaHSO3��Һ�У��й�����Ũ���ɴ�С��˳��Ϊ��
c ��Na+����c ��HSO3������c ��SO32������c ��H2SO3��
�ٸ���Һ��c ��H+�� c ��OH������������������� ���� �����������ǣ������ӷ���ʽ��ʾ���� ��
������NaHSO3��Һ�У���μ����������з�̪��NaOH��Һ���ɹ۲쵽�������� ����Ӧ�����ӷ���ʽΪ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com