
ŌŚ»ØĘæÖŠ¼ÓČė”°ĻŹ»Ø±£ĻŹ¼Į”±£¬ÄÜŃÓ³¤ĻŹ»ØµÄŹŁĆü”£ĻĀ±ķŹĒ500mL”°ĻŹ»Ø±£ĻŹ¼Į”±ÖŠŗ¬ÓŠµÄ³É·Ö£¬ŌĶĮŗó»Ų“šĻĀĮŠĪŹĢā£ŗ
| ³É·Ö | ÖŹĮæ£Øg£© | Ħ¶ūÖŹĮæ£Øg”¤mol-1£© |
| ÕįĢĒ | 25.00 | 342 |
| ĮņĖį¼Ų | 0.87 | 174 |
| °¢Ė¾Ę„ĮÖ | 0.17 | 180 |
| øßĆĢĖį¼Ų | 0.316 | 158 |
| ĻõĖįŅų | 0.075 | 170 |
 ³¬ÄÜѧµäµ„ŌŖĘŚÖŠĘŚÄ©×ØĢā³å“Ģ100·ÖĻµĮŠ“š°ø
³¬ÄÜѧµäµ„ŌŖĘŚÖŠĘŚÄ©×ØĢā³å“Ģ100·ÖĻµĮŠ“š°ø »ĘøŌ360¶Č¶ØÖĘĆܾķĻµĮŠ“š°ø
»ĘøŌ360¶Č¶ØÖĘĆܾķĻµĮŠ“š°ø Ńō¹āæ¼³”µ„ŌŖ²āŹŌ¾ķĻµĮŠ“š°ø
Ńō¹āæ¼³”µ„ŌŖ²āŹŌ¾ķĻµĮŠ“š°ø ĆūŠ£ĮŖĆĖ³å“Ģ¾ķĻµĮŠ“š°ø
ĆūŠ£ĮŖĆĖ³å“Ģ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ij¹ĢĢå»ģŗĻĪļŗ¬NH4I”¢NaHCO3”¢AlCl3”¢MgBr2”¢FeCl2ÖŠµÄ¼øÖÖ,ĪŖČ·¶ØøĆ¹ĢĢå»ģŗĻĪļµÄ³É·Ö¼°ø÷×é³É³É·ÖµÄĪļÖŹµÄĮæÖ®±Č,ĻÖ½ųŠŠČēĻĀŹµŃ锣
ŹµŃé¢ń:
(1)ĪŽÉ«ĘųĢåĪŖ ”£
(2)øĆ¹ĢĢå»ģŗĻĪļµÄ³É·ÖĪŖ ”£
ŹµŃé¢ņ:Č”Ņ»¶ØĮæµÄøĆ¹ĢĢå»ģŗĻĪļČÜÓŚĖ®Åä³É1 LČÜŅŗ,²¢ĻņøĆ»ģŗĻČÜŅŗÖŠĶØČėŅ»¶ØĮæµÄCl2,²āµĆČÜŅŗÖŠ¼øÖÖŅõĄė×Ó(·Ö±šÓĆA-”¢B-”¢C-±ķŹ¾)µÄĪļÖŹµÄĮæÓėĶØČėCl2Ģå»żµÄ¹ŲĻµČē±ķĖłŹ¾”£
| Cl2µÄĢå»ż (±ź×¼×“æöĻĀ)/L | 2.8 | 5.6 | 11.2 |
| n(A-)/mol | 1.25 | 1.5 | 2 |
| n(B-)/mol | 1.5 | 1.4 | 0.9 |
| n(C-)/mol | a | 0 | 0 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©
£Ø1£©ÖŹĮæĻąĶ¬µÄO2”¢NH3”¢H2”¢Cl2ĖÄÖÖĘųĢåÖŠ£¬ŌŚĻąĶ¬ĪĀ¶ČŗĶĻąĶ¬Ń¹ĒæĢõ¼žĻĀ£¬Ģå»ż×ī“óµÄŹĒ ”£
£Ø2£©ĻĀĮŠĪļÖŹÖŠ£¬¼ČÄÜÓėŃĪĖį·“Ó¦£¬ÓÖÄÜÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄŹĒ_______”££ØĢīŠņŗÅ£©
¢ŁNaAlO2 ¢Ś Ca(OH)2 ¢ŪNa2CO3 ¢ÜAl(OH)3
£Ø3£©ĀČĖ®ÖŠŗ¬ÓŠ¶ąÖֳɷ֔£½«×ĻÉ«ŹÆČļŹŌŅŗµĪČėĀČĖ®ÖŠ£¬ČÜŅŗĻŌŗģÉ«Ęš×÷ÓĆµÄ³É·ÖŹĒ
£»¹żŅ»»į¶ł£¬ČÜŅŗŃÕÉ«Öš½„ĶŹČ„£¬Ęš×÷ÓĆµÄ³É·ÖŹĒ £»
£Ø4£©±ź×¼×“æöĻĀ°Ń11.2LĀČĘųĶØČė500ml0.8mol/LFeBr2ČÜŅŗÖŠ£¬Š“³ö·“Ó¦ĶźČ«ŗóµÄĄė×Ó·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ķ¬ĪĀĶ¬Ń¹ĻĀ£¬ÓŠ0.3molO2Óė0.2molO3£¬ĖüĆĒµÄÖŹĮæÖ®±ČĪŖ £¬ĖüĆĒĖłŗ¬µÄŌ×ÓŹżÖ®±ČĪŖ £¬ĖüĆĒµÄĢå»żÖ®±ČĪŖ £¬ĖüĆĒµÄĆܶČÖ®±ČĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼ĪŖŹµŃéŹŅijÅØĮņĖįŹŌ¼ĮĘæÉĻµÄ±źĒ©£¬ŹŌøł¾ŻÓŠ¹ŲŹż¾Ż»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©øĆÅØĮņĖįµÄĪļÖŹµÄĮæÅضČĪŖ__________mol/L”£
£Ø2£©Č”ÓĆČĪŅāĢå»żµÄøĆĮņĖįČÜŅŗŹ±£¬ĻĀĮŠĪļĄķĮæÖŠ²»ĖęĖłČ”Ģå »żµÄ¶ąÉŁ¶ų±ä»ÆµÄŹĒ__________”£
A£®ČÜŅŗÖŠH2SO4µÄĪļÖŹµÄĮæ B£®ČÜŅŗµÄÅضČ
C£®ČÜŅŗÖŠSO42£µÄŹżÄæ D£®ČÜŅŗµÄĆܶČ
£Ø3£©Ä³Ń§ÉśÓūÓĆÉĻŹöÅØĮņĖįŗĶÕōĮóĖ®ÅäÖĘ480 mLĪļÖŹµÄĮæÅضČĪŖ0.2 mol/LĻ”ĮņĖį”£
¢ŁøĆѧɜŠčŅŖĮæČ”________mLÉĻŹöÅØĮņĖį½ųŠŠÅäÖĘ”£
¢ŚÅäÖĘŹ±£¬ĘäÕżČ·µÄ²Ł×÷Ė³ŠņŹĒ£Ø×ÖÄø±ķŹ¾£¬Ćæøö×ÖÄøÖ»ÄÜÓĆŅ»“Ī£©________________£»
A£®ÓĆ30mLĖ®Ļ“µÓÉÕ±2”«3“Ī£¬Ļ“µÓŅŗ¾ł×¢ČėČŻĮæĘ棬Õńµ“
B£®ÓĆĮæĶ²×¼Č·ĮæČ”ĖłŠčÅØĮņĖįµÄĢå»ż£¬ĀżĀżŃŲ±±Ś×¢ČėŹ¢ÓŠÉŁĮæĖ®£ØŌ¼30mL£©µÄÉÕ±ÖŠ£¬ÓĆ²£Į§°ōĀżĀż½Į¶Æ£¬Ź¹Ęä»ģŗĻ¾łŌČ
C£®½«ŅŃĄäČ“µÄĮņĖįŃŲ²£Į§°ō×¢ČėŅ»¶ØĢå»żµÄČŻĮæĘæÖŠ
D£®½«ČŻĮæĘæøĒ½ō£¬µßµ¹Ņ”ŌČ
E£®øÄÓĆ½ŗĶ·µĪ¹Ü¼ÓĖ®£¬Ź¹ČÜŅŗ°¼ĆęĒ”ŗĆÓėæĢ¶ČĻßĻąĒŠ
F£®¼ĢŠųĶłČŻĮæĘæÄŚŠ”ŠÄ¼ÓĖ®£¬Ö±µ½ŅŗĆę½Ó½üæĢ¶ČĻß1”«2cm“¦
¢ŪŌŚÅäÖĘ¹ż³ĢÖŠ£¬ĻĀĮŠŹµŃé²Ł×÷Ź¹ĖłÅäÖʵÄĻ”ĮņĖįµÄĪļÖŹµÄĮæÅضČĘ«øߵďĒ_________
A£®ÓĆĮæĶ²ĮæČ”ÅØĮņĖįŹ±ŃöŹÓ¹Ū²ģ°¼ŅŗĆę
B£®Ļ”ŹĶÓƵÄÉÕ±ŗĶ²£Į§°ōĪ“Ļ“µÓ
C£®Ļ“¾»µÄČŻĮæĘæĪ“¾øÉŌļ¾ĶÓĆÓŚÅäÖĘČÜŅŗ
D£®ČÜŅŗ×¢ČėČŻĮæĘæĒ°Ć»ÓŠ»Öø“µ½ŹŅĪĀ¾Ķ½ųŠŠ¶ØČŻ
E£®¶ØČŻŹ±ø©ŹÓ¹Ū²ģ°¼ŅŗĆę
F£®¼ÓĖ®³¬¹żæĢ¶ČĻßŗó£¬ÓĆ½ŗĶ·µĪ¹ÜĪü³ö¶ąÓąµÄŅŗĢå
¢ÜĻÖ½«100mLøĆĮņĖįÓė300mL 0.4mol/LCuSO4ČÜŅŗ»ģŗĻ£¬Ģå»ż±ä»ÆŗöĀŌ²»¼Ę£¬ĖłµĆČÜŅŗÖŠSO42£µÄĪļÖŹµÄĮæÅØ¶ČŹĒ_________mol/L”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŌŚ300 mL2mol/LH2SO4ČÜŅŗÖŠ£¬ČÜÖŹµÄÖŹĮæŹĒ £¬“ĖČÜŅŗÖŠŗ¬H+µÄĪļÖŹµÄĮæŹĒ £¬H+µÄĪļÖŹµÄĮæÅØ¶ČŹĒ £¬ĘäÖŠŗ¬ÓŠ øöSO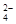 £¬SO
£¬SO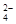 µÄĪļÖŹµÄĮæÅØ¶ČŹĒ ”£
µÄĪļÖŹµÄĮæÅØ¶ČŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĒėĶź³ÉĻĀĮŠĢīæÕ£ŗ
£Ø1£©µ±SO2ŗĶSO3ÖŠ·Ö×ÓøöŹż±ČĪŖ1”Ć1 Ź±£¬Ō×Ó×ÜŹżÖ®±ČĪŖ £¬ÖŹĮæÖ®±ČĪŖ ”£
£Ø2£©ÖŠŗĶŗ¬0.2 mol HClµÄĻ”ŃĪĖį£¬ŠčNaOHµÄÖŹĮæĪŖ g”£
£Ø3£©ĻÖÓŠm gijĘųĢ壬ĖüÓÉĖ«Ō×Ó·Ö×Ó¹¹³É£¬ĖüµÄĦ¶ūÖŹĮæĪŖM g”¤mol-1£¬Ōņ£ŗ
¢ŁøĆĘųĢåµÄĪļÖŹµÄĮæĪŖ mol”£
¢ŚøĆĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ L”£
¢ŪøĆĘųĢåČÜÓŚĖ®ŗóŠĪ³ÉV LČÜŅŗ£Ø²»æ¼ĀĒ·“Ó¦£©£¬ĘäČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ mol/L”£
£Ø4£©½«5mol/LµÄMg(NO3)2ČÜŅŗa mLĻ”ŹĶÖĮb mL£¬Ļ”ŹĶŗóČÜŅŗÖŠNO3-µÄĪļÖŹµÄĮæÅØ¶ČŹĒ mol/L”£
£Ø5£©ÓƵČĢå»żµÄ0.1mol/LµÄBaCl2ČÜŅŗ£¬æÉŹ¹ĻąĶ¬Ģå»żµÄFe2(SO4)3”¢Na2SO4”¢KAl(SO4)2ČżÖÖČÜŅŗÖŠµÄSO42-Ē”ŗĆĶźČ«³Įµķ£¬ŌņČżÖÖĮņĖįŃĪµÄĪļÖŹµÄĮæÅضČÖ®±ČĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¹żŃõŅŅĖįŹĒŅ»ÖÖČõĖįŠŌŃõ»Æ¼Į£¬¹ć·ŗÓĆ×÷ĘÆ°×¼ĮŗĶøߊ§É±¾śĻū¶¾¼ĮµČ”£
¢ń”¢Öʱø£ŗ±ł“×ĖįÓėH2O2ÅØČÜŅŗ°“Ģå»ż±Č1:1»ģŗĻ£¬¼ÓČėŹŹĮæÅØĮņĖį£¬æŲÖĘĪĀ¶Č5”ꔫ30”ę£¬½Į°č30min²¢¾²ÖĆ4”«6h”£
·“Ó¦ŌĄķĪŖ£ŗH2O2+CH3COOH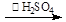
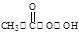 £Ø¹żŃõŅŅĖį£©+H2O
£Ø¹żŃõŅŅĖį£©+H2O
¢ņ”¢ŗ¬Įæ²ā¶Ø£ŗ³ĘČ”5.0000g¹żŃõŅŅĖįŹŌŃł£ØŅŗĢ壩£¬Ļ”ŹĶÖĮ100mL±øÓĆ”£Č”ÉĻŹöĻ”ŹĶŗó¹żŃõŅŅĖįŹŌŃł5.00mL£¬ÓĆ0.0100 mol”¤L£1KMnO4ČÜŅŗµĪ¶Øµ½ÖÕµćŅŌ³żČ„ĘäÖŠµÄH2O2£¬Ėę¼“¼ÓČė10%KIČÜŅŗ10mL£¬¼ÓČė0.5%µķ·ŪČÜŅŗ3µĪ£¬Ņ”ŌČ£¬²¢ÓĆ0.0500 mol”¤L£1Na2S2O3±ź×¼ČÜŅŗµĪ¶Øµ½Ą¶É«øÕŗĆĶŹČ„£ØĄė×Ó·“Ó¦·½³ĢŹ½ĪŖ£ŗI2+2S2O32£=2I£+S4O62££©£¬ĻūŗÄNa2S2O3±ź×¼ČÜŅŗµÄ×ÜĢå»żĪŖ20.00mL”£
£Ø1£©Öʱø¹żŃõŅŅĖįŹ±£¬ĪĀ¶Č²»ŅĖ¹żøߣ¬ĘäŌŅņæÉÄÜŹĒ ”£
£Ø2£©Ļ”ŹĶѳʷŹ±£¬³żÓƵ½ČŻĮæĘæ¼°ÉÕ±Ķā£¬»¹ÓƵ½µÄ²£Į§ŅĒĘ÷ÓŠ ”¢ ”£
£Ø3£©¹żŃõŅŅĖįČÜŅŗÓėKI·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø4£©Ķعż¼ĘĖćČ·¶ØŌŹŌŃłÖŠ¹żŃõŅŅĖįµÄÖŹĮæ·ÖŹż(Š“³ö¼ĘĖć¹ż³Ģ)”£ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
½«50.0gŗ¬ÓŠNH4NO3”¢£ØNH4£©2SO4¼°ĘäĖüÄŃČÜĪļµÄ»ģŗĻĪļѳʷ£¬ČÜÓŚĖ®£¬¹żĀĖŗó¼ÓČė×ćĮæÅØNaOHČÜŅŗ¹²ČČ£¬ŌŚ±ź×¼×“æöĻĀŹÕ¼Æµ½13.44 L°±Ęų£¬ŌŁĻņČÜŅŗÖŠ¼ÓČė×ćĮæBaCl2ČÜŅŗ£¬²śÉś³Įµķ46.6g£¬ŹŌ¼ĘĖć»ģŗĻĪļÖŠNH4NO3µÄĪļÖŹµÄĮæ¼°£ØNH4£©2SO4µÄÖŹĮæ·ÖŹż”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com