

��1����֪��ȩ��ȼ����Ϊ1 815 kJ��mol-1����ͪ��ȼ����Ϊ1789 kJ��mol-1����д����ȩȼ�յ��Ȼ�ѧ����ʽ_________________��
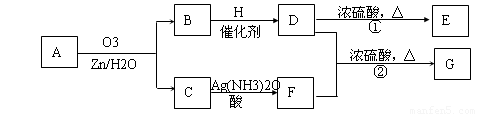
��2��������Ӧ�������ƶ�ϩ���Ľṹ��һ����״��ϩ��Aͨ��������������п��ˮ�����õ�B��C��������B��̼69.8%������11.6%��B��������Ӧ������������D��D��Ũ��������¼��ȣ��ɵõ���ʹ��ˮ��ɫ��ֻ��һ�ֽṹ������E����Ӧͼʾ���£�

����������⣺
��B����Է���������__________��
C![]() F�ķ�Ӧ����Ϊ__________��
F�ķ�Ӧ����Ϊ__________��
D�к��й����ŵ�����__________��
��D+F![]() G�Ļ�ѧ����ʽ�ǣ�_________________��
G�Ļ�ѧ����ʽ�ǣ�_________________��
��A�Ľṹ��ʽΪ___________________________________��
�ܻ�����A��ij��ͬ���칹��ͨ��������������п��ˮ����ֻ�õ�һ�ֲ�����ϸ��������칹��Ľṹ��ʽ��__________�֡�
��������1����д�Ȼ�ѧ����ʽʱ������Ӧ����Ȼ�ѧ����ʽ�Ķ��壺1 mol������ȫȼ�������ȶ���������ʱ���ų����������Ӷ�д����Ӧ���Ȼ�ѧ����ʽ��
��2���ٸ���C��H��������������������������������������������B�к���һ����ԭ�ӣ��Ӷ�ȷ��B����Է�������Ϊ86������ʽΪC5H10O��B��������Ӧ��˵��BΪͪ��B��ԭ��õ�����D����D��ˮ������ϩ��ֻ��һ�ֽṹ����ṹ��ʽΪCH3CH��DΪ3-�촼����F�����ں���8��̼ԭ�ӿ�֪��C��F�ж�����3��̼ԭ�ӣ�CΪ��ȩ��FΪ���ᣬ�Ӷ��ó���
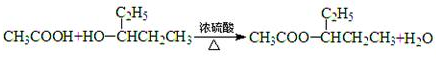
��CH3CH2COOH+��C2H5��2CHOH![]() CH3CH2COOCH��C2H5��2+H2O
CH3CH2COOCH��C2H5��2+H2O
�۾������Ϸ����������ó�A�Ľṹ��ʽΪ��CH3CH2��2C=CHCH2CH3��
��A�ķ���ʽΪC8H16��������֪A�ķ��ӽṹ�ǶԳƵģ��Ӷ��ó����ܽṹΪ3�֡�
�𰸣���1��CH3CH2CHO(l)+4O2(g)![]() 3CO2(g)+3H2O(l);��H=-1 815 kJ��mol-1
3CO2(g)+3H2O(l);��H=-1 815 kJ��mol-1
��2����86 ������Ӧ �ǻ�
��CH3CH2COOH+��C2H5��2CHOH![]() CH3CH2COOCH(C2H5)2+H2O
CH3CH2COOCH(C2H5)2+H2O
��(CH3CH2)2C=CHCH2CH3
��3



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
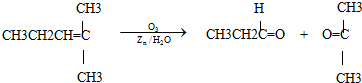
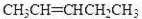
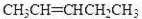

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���Ϻ���ʮУ������ѧ�ڵڶ���������ѧ�Ծ� ���ͣ������
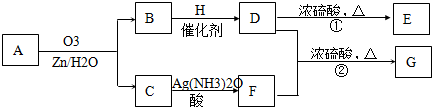
ϩ��ͨ��������������п��ˮ�����õ�ȩ��ͪ�����磺
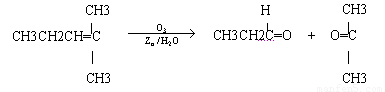
һ����״��ϩ��Aͨ��������������п��ˮ�����õ�B��C��������B��̼69��8��������11��6����B��������Ӧ��D��Ũ��������¼��ȣ��ɵõ���ʹ��ˮ��ɫ��ֻ��һ�ֽṹ������E��G�ķ���ʽΪC7H14O2���й�ת����ϵ���£�
��1��B����Է���������__________________��
��2��д���ṹ��ʽ��A__________________��E_______________________��
��3��д����Ӧ�١��ڵķ�Ӧ���ͣ���________________����_______________��
��4��д����Ӧ�ڵĻ�ѧ����ʽ��__________________________________________��
��5��F��һ��ͬ���칹���ܷ���������Ӧ��������Ӧ����ṹ��ʽΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ϩ��ͨ��������������п��ˮ�����õ�ȩ��ͪ�����磺
һ����״��ϩ��Aͨ��������������п��ˮ�����õ�B��C��������B��̼69��8��������11��6����B��������Ӧ��D��Ũ��������¼��ȣ��ɵõ���ʹ��ˮ��ɫ��ֻ��һ�ֽṹ������E��G�ķ���ʽΪC7H14O2���й�ת����ϵ���£�
��1��B����Է���������__________________��
��2��д���ṹ��ʽ��A__________________��E_______________________��
��3��д����Ӧ�١��ڵķ�Ӧ���ͣ���________________����_______________��
��4��д����Ӧ�ڵĻ�ѧ����ʽ��__________________________________________��
��5��F��һ��ͬ���칹���ܷ���������Ӧ��������Ӧ����ṹ��ʽΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���Ϻ���ʮУ������ѧ�ڵڶ���������ѧ�Ծ� ���ͣ������
ϩ��ͨ��������������п��ˮ�����õ�ȩ��ͪ�����磺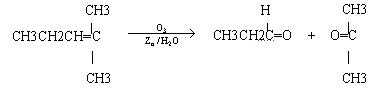
һ����״��ϩ��Aͨ��������������п��ˮ�����õ�B��C��������B��̼69��8��������11��6����B��������Ӧ��D��Ũ��������¼��ȣ��ɵõ���ʹ��ˮ��ɫ��ֻ��һ�ֽṹ������E��G�ķ���ʽΪC7H14O2���й�ת����ϵ���£�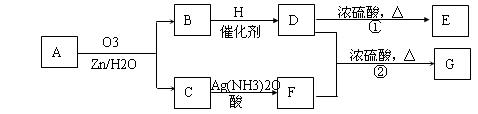
��1��B����Է���������__________________��
��2��д���ṹ��ʽ��A__________________��E_______________________��
��3��д����Ӧ�١��ڵķ�Ӧ���ͣ���________________����_______________��
��4��д����Ӧ�ڵĻ�ѧ����ʽ��__________________________________________��
��5��F��һ��ͬ���칹���ܷ���������Ӧ��������Ӧ����ṹ��ʽΪ__________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com