
| A�� | 0.1mol/L NaHCO3��Һ��0.1mol/L NaOH��Һ�������ϣ�������Һ��c��Na+����c��CO32-����c��HCO3- ����c��OH-�� | |
| B�� | pH��ȵĢ�NH4Cl �ڣ�NH4��2SO4 ��NH4HSO4��Һ�У�c��NH4+����С˳��=�ڣ��� | |
| C�� | �����£�pH=2��������pH=12�İ�ˮ�������ϣ�������Һ�У�c��Cl-����c��H+����c��NH4+ ����c��OH-�� | |
| D�� | 0.2mol•L-1CH3COOH��Һ��0.1mol•L-1NaOH��Һ�������ϣ�2c��H+��-2c��OH-���TC��CH3COO-��+C��CH3COOH�� |
���� A��0.1mol/L NaHCO3�ܣ�����Ũ��Һ��0.1mol/L NaOH��Һ�������Ϸ�Ӧ�õ�̼������Һ������Ũ�ȴ�С����̼������Һ��̼�������ˮ�������
B���������Ƕȿ��ǣ�һ����ͬpH�ģ�NH4��2SO4��NH4Cl��Һ��ˮ��̶��Ƿ���ȣ�����NH4HSO4����ʱ����H+ʹ��Һ�����ԣ�NH4+��ˮ�ⱻ���ƣ��Դ������
C��pH=12�İ�ˮ��pH=2�������У���ˮŨ�ȴ������ᣬ�������ϣ���ˮ��������Һ�ʼ��ԣ�
D��0.2mol•L-1CH3COOH��Һ��0.1mol•L-1NaOH��Һ�������ϵõ���Ũ�ȵ�CH3COOH��CH3COONa����Һ�����������ڴ��������ˮ��̶ȣ�������Һ�е���غ㡢�����غ���������
��� �⣺A��0.1mol/L NaHCO3�ܣ�����Ũ��Һ��0.1mol/L NaOH��Һ�������Ϸ�Ӧ�õ�̼������Һ��̼������ӷֲ�ˮ�⣬��Һ�Լ��ԣ�����Ũ�ȴ�СΪ��c��Na+����c��CO32-����c��OH-����c��HCO3- ������A����
B����ͬpH�ģ�NH4��2SO4��NH4Cl��Һ�У�����ǿ�������Σ�������Һ�ʵ����Կ��ж϶���NH4+Ũ����ȣ�����NH4HSO4����ʱ����H+ʹ��Һ�����ԣ�NH4+��ˮ�ⱻ���ƣ����NH4HSO4��NH4+��Ũ��С�ڣ�NH4��2SO4��ӦΪ����=�ڣ��ۣ���B��ȷ��
C��pH=12�İ�ˮ��pH=2�������У���ˮŨ�ȴ������ᣬ�������ϣ���ˮ��������Һ�ʼ��ԣ�����c��OH-����c��H+������Һ�д��ڵ���غ�c��NH4+��+c��H+��=c��Cl-��+c��OH-�������Ե�c��NH4+����c��Cl-������Һ��һˮ�ϰ�����̶Ƚ�С������c��Cl-����c��OH-��������Һ������Ũ�ȴ�С˳����c��NH4+����c��Cl-����c��OH-����c��H+������C����
D��0.2mol•L-1CH3COOH��Һ��0.1mol•L-1NaOH��Һ�������ϵõ���Ũ�ȵ�CH3COOH��CH3COONa����Һ�����������ڴ��������ˮ��̶ȣ���Һ�д��ڵ���غ�Ϊ��c��H+��+c��Na+���TC��CH3COO-��+c��OH-������Һ�е������غ�õ�2c��Na+���TC��CH3COO-��+C��CH3COOH��������õ�2c��H+��-2c��OH-���TC��CH3COO-��-C��CH3COOH������D����
��ѡB��
���� ���⿼��������Ũ�ȴ�С�ıȽϵ�֪ʶ�㣬��ȷ������ʵ��뼰����ˮ���ص��ǽⱾ��ؼ����ٽ�������غ㡢����غ�ȷ������Ũ�ȴ�С���ѵ�������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ�


 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CuCl2 NH4Cl Fe2��SO4��3 NaNO3 | |
| B�� | ��NH4��2SO4 CuSO4 Na2CO3 FeCl3 | |
| C�� | Cu��NO3��2 NH4NO3 FeCl3 H2S | |
| D�� | Ba Cl2 Cu��NO3��2 ��NH4��2SO4 Fe ��NO3��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO3 | B�� | PF5 | C�� | CCl4 | D�� | NO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | �ԡ��ع��͡�������Ի�����͵�ȼ�� | |
| B�� | �������������ټ����õĺ������ơ��Ȼ����Լ�̼��ƶ������� | |
| C�� | �������ھ�ˮ���������ˮ���йأ����뽺��������й� | |
| D�� | FeS������ˮ��Ksp=1.6��10-19�������ܳ�ȥˮ�е�Cu2+[��֪Ksp��CuS��=6.3��10-36] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ˮƿ��ʱ�д�������ð����������������ԭ������ | |
| B�� | ���ȵĴ��Na2CO3����Һ����ϴȥ�;��ϵ����� | |
| C�� | ��ţ���м����֭�����������������Ϊ�������кͷ�Ӧ | |
| D�� | ����������ˮ����ͭ��ˮ��ͷ����ʹ��ʱ�����Ӵ�����ˮ����ʴ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 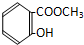 | B�� | 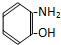 | C�� | 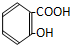 | D�� | 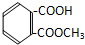 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H278Se���ȶ��Ա�H2S�ȶ���ǿ | |
| B�� | ${\;}_{34}^{78}$Se��${\;}_{34}^{80}$Se��Ϊͬ�������� | |
| C�� | ${\;}_{34}^{78}$Se��${\;}_{34}^{80}$Se��ɵ���̬�������ڻ����� | |
| D�� | ${\;}_{34}^{78}$Se��${\;}_{34}^{80}$Se��Ϊͬλ�أ��ֱ���44��46������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com