
CH4��H2��CO��ȼ���ȷֱ�Ϊ890.31kJ/mol��285.8kJ/mol��110.5 kJ/mol�������Ȼ�ѧ����ʽ��д��ȷ����
A��CH4(g)+2O2(g)=CO2(g)+2H2O(g) ��H=-890.31kJ/mol
B��2H2(g)+ O2(g)= 2H2O(l) ��H=-285.8kJ/mol
 C��CO (g)+ H2O(g)= CO2(g)+
C��CO (g)+ H2O(g)= CO2(g)+  H2 (g) ��H=+175.3kJ/mol
H2 (g) ��H=+175.3kJ/mol
D��2CO (g)+ O2(g) = 2CO2(g) ��H=-221 kJ/mol

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2Na2O2+2H2O=4NaOH+O2��������˵����ȷ���� �� ��
A��ÿ����224mLO2��ת��0.01mol e-
B���������Ƶ�Ħ������Ϊ78g
C. ��0.01mol Na2O2���뵽ˮ�еõ�100mL��Һ���������ʵ���Ũ��Ϊ0.2mol/L
D����Na2O2���������Ӹ�����Ϊ1:1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K����NH ��Cl����Mg2����Ba2����CO
��Cl����Mg2����Ba2����CO ��SO
��SO ����ȡ����100 mL��Һ��������ʵ�飺
����ȡ����100 mL��Һ��������ʵ�飺
(1)��һ�ݼ���AgNO3��Һ�г���������
(2)�ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.04 mol��
(3)�����ݼ�����BaCl2��Һ�ø������6.27 g������������ϴ�ӡ������������Ϊ2.33 g��
��������ʵ�飬�����Ʋ���ȷ����(����)
A��K����һ������
B��100 mL��Һ�к�0.01 mol CO
C��Cl�����ܴ���
D��Ba2��һ�������ڣ�Mg2�����ܴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڲ�ͬ״̬�£���̬����̬����������Ӧ���Ȼ�ѧ����
ʽ������ʾ��
��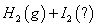 =
=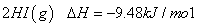
��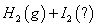 =
=
�����ж���ȷ����
A�����е�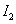 Ϊ��̬�����е�
Ϊ��̬�����е�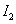 Ϊ��̬
Ϊ��̬
B���ڵķ�Ӧ���������Ȣٵķ�Ӧ����������
C���ٵIJ���ȷ�Ӧ�ڵIJ������ȶ��Ը���
D��1mol��̬������ʱ������17kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̼���γɻ�������������Ԫ�أ��䵥�ʼ������������������������Ҫ��Դ���ʡ���ش��������⣺
��1���л���M����̫������տ�ת����N��ת���������£�

 ����H����88.6 kJ·mol��1����M��N��ȣ����ȶ�����____________��
����H����88.6 kJ·mol��1����M��N��ȣ����ȶ�����____________��
��2����֪CH3OH(l)��ȼ���� Ϊ238.6 kJ·mol��1��CH3OH(l)��
Ϊ238.6 kJ·mol��1��CH3OH(l)�� O2(g)===CO2(g)��2H2(g)����H����a kJ·mol��1����a______238.6(�>������<������)��
O2(g)===CO2(g)��2H2(g)����H����a kJ·mol��1����a______238.6(�>������<������)��
��3��ʹCl2��H2O(g)ͨ�����ȵ�̿�㣬����HCl��CO2������1 mol Cl2���뷴Ӧʱ�ͷų�145 kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��______________________________��
��4������͵�������ı������������ʡ���ʯī�����ۺͶ������Ѱ�һ����������ڸ��������գ��������ʿ������²��ϣ�4Al(s)��3TiO2(s)��3C(s)===2Al2O3(s)��3TiC(s)����H����1 176 kJ·mol��1����Ӧ�����У�ÿת��1 mol���ӷų�������Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Դ�ǹ��÷�չ����Ҫ�������ҹ�Ŀǰʹ�õ���Դ��Ҫ�ǻ�ʯȼ�ϡ�
��1����25 �桢101 kPaʱ��16 g CH4��ȫȼ������Һ̬ˮʱ�ų���������890.31 kJ����CH4ȼ�յ��Ȼ�ѧ����ʽ��________________________________��
��2����֪��C��s����O2��g��===CO2��g������H����437.3 kJ·mol��1
H2��g���� O2��g��===H2O��g������H����285.8 kJ·mol��1
O2��g��===H2O��g������H����285.8 kJ·mol��1
CO��g���� O2��g��===CO2��g������H����283.0 kJ·mol��1
O2��g��===CO2��g������H����283.0 kJ·mol��1
��ú��������Ҫ��Ӧ���Ȼ�ѧ����ʽ��
C��s����H2O��g��===CO��g����H2��g������H��________kJ·mol��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ�����ö������棨CeO2����̫���������½�H2O��CO2ת���H2��CO����������£�
mCeO2 ��m-x��CeO2·xCe+xO2
��m-x��CeO2·xCe+xO2
��m-x��CeO2·xCe+xH2O+ xCO2 mCeO2+ xH2+ xCO
mCeO2+ xH2+ xCO
 ����˵������ȷ����
����˵������ȷ����
A���ù�����CeO2û������
B���ù���ʵ����̫������ѧ�ܵ�ת��
C����ͼ�С�H1=��H2+��H3
D����CO��O2���ɵļ���ȼ�ϵ�صĸ�����ӦʽΪ
CO+4OH——2e—=CO +2H2O
+2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ��ʹNa2S��Һ��[Na��]/[S2��]�ı�ֵ��С���ɲ�ȡ�Ĵ�ʩ��(����)
�ټ����������ᡡ�ڼ�������NaOH(s)���ۼ�������KOH(s)���ܼ�������KHS(s)��
�ݼ�ˮ����ͨH2S(g)������
A���ڢۢ� B���٢ڢݢޢ� C���ڢ� D���ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ե��з�̪��Һ��������Һ����������ɫ�������
A��������Һ���� B��CH3COONa��Һ����
C����ˮ�м�������NH4Cl���� D��С�մ���Һ�м�������NaCl����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com