


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(6��)�Ķ���������������Ϣ���ش����⣺
��1��һ������50Kg�Ľ����˺���2g����2g���������в����Ե��ʵ���ʽ���ڣ�������Fe2+��Fe3+����ʽ���ڡ����������ױ����գ���ƶѪ�߲�����ʱ��Ӧ���躬�������ӵ������Σ�����������������ά����C����ʹʳ���е������ӻ�ԭ���������ӣ��������������ա�
��2�����������ĺ�ˮ����һ����ϸ����������ȡ��ˮ�е��������ӣ�����øΪ��������ת������ǵ�Ƥ�ʣ�������Fe2O3����ʾ���е���������������������γ����÷�Ӧ����һ�ַ�Ӧ����CO2����Ӧ��CO2ת����л���ü�ȩ��CH2O��ʾ����
���⣺
�����������Ϣ�ֱ�����������ںͺ�ˮ�е����������������ӣ��������ĸۻ�����ת������ʵ��������;������˵����������(Fe2+)���� �ԣ�������(Fe3+)���� �ԣ�
��1���е�ά����C�� ������2���е�CO2 ����д����2���е����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)���������ַ��������Ƶð�ɫ��Fe(OH)2������
����һ���ò���Fe3+��FeSO4��Һ���ò���O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ���
��1������������������������FeSO4��Һʱ�������ϡH2SO4��______��
��2����ȥ����ˮ���ܽ��O2������____________�ķ�����
��3�����ɰ�ɫFe(OH)2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ�����ټ���NaOH��Һ������������������_______________________________��
������������ͼ��ʾװ���У���NaOH��Һ����м��ϡH2SO4���Լ��Ʊ���
��1�����Թܹ��������Լ���_______________��
��2�����Թܢ��������Լ���_______________��
��3��Ϊ���Ƶð�ɫFe(OH)2���������Թܢ�͢��м����Լ�����ֹˮ�У��������Ӻ��ʵ�鲽����_________________________________��
��4���������ɵ�Fe(OH)2�����ܽϳ�ʱ�䱣�ְ�ɫ����������________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���¿α껯ѧ����һ�����½������仯���ﵥԪ���ԣ��˽̰棩 ���ͣ������
(12��)���������ַ��������Ƶð�ɫ��Fe(OH)2������
����һ���ò���Fe3+��FeSO4��Һ���ò���O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ���
��1������������������������FeSO4��Һʱ�������ϡH2SO4��______��
��2����ȥ����ˮ���ܽ��O2������____________�ķ�����
��3�����ɰ�ɫFe(OH)2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ�����ټ���NaOH��Һ������������������_______________________________��
������������ͼ��ʾװ���У���NaOH��Һ����м��ϡH2SO4���Լ��Ʊ���
��1�����Թܹ��������Լ���_______________��
��2�����Թܢ��������Լ���_______________��
��3��Ϊ���Ƶð�ɫFe(OH)2���������Թܢ�͢��м����Լ�����ֹˮ�У��������Ӻ��ʵ�鲽����_________________________________��
��4���������ɵ�Fe(OH)2�����ܽϳ�ʱ�䱣�ְ�ɫ����������________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���¿α껯ѧ����һ�����½������仯���ﵥԪ���ԣ��˽̰棩 ���ͣ������
(12��)���������ַ��������Ƶð�ɫ��Fe(OH)2������
����һ���ò���Fe3+��FeSO4��Һ���ò���O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ���
��1������������������������FeSO4��Һʱ�������ϡH2SO4��______��
��2����ȥ����ˮ���ܽ��O2������____________�ķ�����
��3�����ɰ�ɫFe(OH)2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ�����ټ���NaOH��Һ������������������_______________________________��
������������ͼ��ʾװ���У���NaOH��Һ����м��ϡH2SO4���Լ��Ʊ���
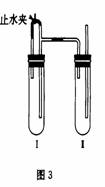
��1�����Թܹ��������Լ���_______________��
��2�����Թܢ��������Լ���_______________��
��3��Ϊ���Ƶð�ɫFe(OH)2���������Թܢ�͢��м����Լ�����ֹˮ�У��������Ӻ��ʵ�鲽����_________________________________��
��4���������ɵ�Fe(OH)2�����ܽϳ�ʱ�䱣�ְ�ɫ����������________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com