
°“ŅŖĒóĶź³ÉĻĀĮŠŠ”Ģā.
(1£©0.5 mol H2OµÄÖŹĮæĪŖ g£¬¹²ÓŠ____________øö·Ö×Ó,___________øöµē×Ó”£
(2) 0.01molijĪļÖŹµÄÖŹĮæĪŖ1.08g£¬Ōņ“ĖĪļÖŹµÄĦ¶ūÖŹĮæĪŖ__________________”£
£Ø3£©ÅäÖĘ50 mL 0.2 mol/L CuSO4ČÜŅŗ£¬ŠčŅŖCuSO4_____________g£»ŠčŅŖCuSO4”¤5H2O _____g”£
£Ø4£©ČēĶ¼ŹĒĪŅŠ£»ÆѧŹµŃéŹŅ“Ó»ÆѧŹŌ¼ĮÉĢµźĀņ»ŲµÄĮņĖįŹŌ¼Į±źĒ©ÉĻµÄ²æ·ÖÄŚČŻ”£
¢ŁøĆÅØĮņĖįµÄĪļÖŹµÄĮæÅضČ_____________£¬
¢ŚÓĆøĆÅØĮņĖįÅäÖĘ200mL1mol/LµÄĻ”ĮņĖį£¬ĮæĶ²ĖłŠčĮæČ”øĆÅØĮņĖįµÄĢå»żŹĒ_________mL”£
(1) 9 0.5NA»ņ3.01x1023 5NA»ņ3.01x1024 (2)108g/mol
(3)1.6 2.5 (4) 18.4mol/L 10.9
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©H2OµÄĦ¶ūÖŹĮæĪŖ18g/mol£¬¹Ź0.5mol H2OµÄÖŹĮæĪŖ0.5mol”Į19g/mol=9g£»¹²ÓŠ·Ö×ÓŹż£ŗ0.5mol”ĮNAmol-1=0.5NA£»Ņ»øöĖ®·Ö×ÓÖŠÓŠ10øöµē×Ó£¬µē×ÓŹż£ŗ0.5mol”Į10”ĮNAmol-1=5NA£¬
¹Ź“š°øĪŖ£ŗ9£»0.5NA»ņ3.01”Į1023£»5NA»ņ3.01”Į1024£»
£Ø2£©ŅĄ¾Żn”ĮM=m¶ØĮæ¹ŲĻµ¼ĘĖćµĆµ½Ä¦¶ūÖŹĮæ£ŗM="m/M=1.08g/0.01mol=" =108g/mol£¬¹Ź“š°øĪŖ£ŗ108g/mol£»
£Ø3£©ÓĆCuSO4ÅäÖĘ50mL0.2mol?L-1CuSO4ČÜŅŗ£¬ŠčŅŖCuSO4ÖŹĮæĪŖ£ŗ0.05”Į0.2mol/L”Į160g/mol=1.6g£»ÓĆCuSO4?5H2OÅäÖĘ50mL0.2mol?L-1CuSO4ČÜŅŗ£¬ŠčŅŖCuSO4?5H2OÖŹĮæĪŖ£ŗ0.05”Į0.2mol/L”Į250g/mol=2.5g£¬¹Ź“š°øĪŖ£ŗ1.6£»2.5£»
(4)ČÜŅŗĻ”ŹĶĒ°ŗóČÜÖŹµÄĪļÖŹµÄĮæ²»±ä£¬ÉčŠčÅØĮņĖįµÄĢå»żĪŖV£¬ŌņÓŠ£ŗ0.2L”Į1mol/L=V”Į18.4mol/L£¬V=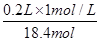 =0.0109L£¬¼“10.9ml£¬¹Ź“š°øĪŖ£ŗ18.4mol/L£»10.9”£
=0.0109L£¬¼“10.9ml£¬¹Ź“š°øĪŖ£ŗ18.4mol/L£»10.9”£
æ¼µć£ŗ°¢·ü¼ÓµĀĀŽ³£Źż£»ĪļÖŹµÄĮæÅØ¶Č£»ĪļÖŹµÄĮæµÄĻą¹Ų¼ĘĖć£»ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¢ń.ŹµŃéŹŅÅäÖĘ1mol/L Na2CO3ČÜŅŗ250ml”£
£Ø1£©ŠčŅŖ¹ĢĢåNa2CO3 g£»£Ø2£©øĆČÜŅŗÖŠµÄŃōĄė×ÓŹżÄæĪŖ øö£»
£Ø3£©ŠčŅŖĪļÖŹµÄĮæÅضČĪŖ5mol/L µÄNa2CO3ČÜŅŗ ml£»
£Ø4£©½«øĆČÜŅŗÓė×ćĮæĻ”ĮņĖį·“Ó¦£¬²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ L”£
£Ø5£©ÅäÖĘøĆČÜŅŗµÄ²Ł×÷Ė³ŠņŹĒ(ÓĆ×ÖÄø±ķŹ¾,²»ÖŲø“Ź¹ÓĆ) ”£
| A£®³ĘĮæ | B£®Ļ“µÓ | C£®¶ØČŻ | D£®Čܽā E£®Ņ”ŌČ F£®×ŖŅĘ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
½«11.2gĢśĶ¶Čė200mLijÅØ¶ČµÄŃĪĖįÖŠ£¬ĢśŗĶŃĪĖįĒ”ŗĆĶźČ«·“Ó¦”£Ēó£ŗ
£Ø1£©ĖłÓĆŃĪĖįÖŠHClµÄĪļÖŹµÄĮæÅضČ
£Ø2£©·“Ó¦ÖŠÉś³ÉµÄH2ŌŚ±ź×¼×“æöĻĀµÄĢå»ż
£Ø3£©ŌŚ·“Ó¦ŗóµÄČÜŅŗÖŠĶØČėCl2£¬Š“³öĖł·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½²¢ÓĆĖ«ĻßĒűź³öµē×Ó×ŖŅʵķ½ĻņŗĶŹżÄæ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŹµŃéŹŅÓĆĀČ»ÆÄĘ¹ĢĢåÅäÖĘ1£®00 mol/LµÄNaClČÜŅŗ0£®5 L£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĒėŠ“³öøĆŹµŃéµÄŹµŃé²½Öč£ŗ
¢Ł £¬¢Ś £¬¢Ū £¬¢Ü £¬¢Ż £¬¢Ž ”£
£Ø2£©ĖłŠčŅĒĘ÷ĪŖ£ŗĶŠÅĢĢģĘ½”¢»¹ŠčŅŖÄĒŠ©ŹµŃéŅĒĘ÷²ÅÄÜĶź³ÉøĆŹµŃ飬ĒėŠ“³ö£ŗ ”£
£Ø3£©ŹŌ·ÖĪöĻĀĮŠ²Ł×÷¶ŌĖłÅäČÜŅŗµÄÅضČÓŠŗĪÓ°Ļģ¼°Ōģ³ÉøĆÓ°ĻģµÄŌŅņ”£
¶ØČŻŗ󣬼ÓøĒµ¹×ŖŅ”ŌČŗ󣬷¢ĻÖŅŗĆęµĶÓŚæĢ¶ČĻߣ¬ÓÖµĪ¼ÓÕōĮóĖ®ÖĮæĢ¶Č”£¶ŌĖłÅäČÜŅŗÅØ¶ČµÄÓ°Ļģ£ŗ £¬ŌŅņŹĒ£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŹµŃéŠčŅŖ0.10mol/LNaOHČÜŅŗ470mL£¬øł¾ŻČÜŅŗÅäÖĘÖŠĒéæö»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŹµŃéÖŠ³żĮĖĶŠÅĢĢģĘ½”¢ÉÕ±”¢²£Į§°ō”¢ĮæĶ²”¢Ņ©³×Ķā»¹ŠčŅŖµÄĘäĖüŅĒĘ÷ÓŠ£ŗ ”£
£Ø2£©øł¾Ż¼ĘĖćµĆÖŖ£¬ĖłŠčNaOHµÄÖŹĮæĪŖ g”£
£Ø3£©¶ØČŻŹ±£¬“żČŻĮæĘæÖŠČÜŅŗµÄ°¼ŅŗĆęÕżŗĆÓėæĢ¶ČĻßĻąĒŠ£¬øĒŗĆĘæČūŗóµÄĻĀŅ»²½²Ł×÷ŹĒ ”£
£Ø4£©¶ØČŻŹ±,Čō¼ÓČėµÄĖ®³¬¹żæĢ¶ČĻߣ¬±ŲŠė²ÉČ”µÄ“ėŹ©ŹĒ£ŗ ”£
£Ø5£©ĻĀĮŠ²Ł×÷¶ŌĖłÅäÅضČÓŠŗĪÓ°Ļģ£ØĢīŠ“×ÖÄø£©
Ę«µĶµÄÓŠ £»ĪŽÓ°ĻģµÄÓŠ ”£
| A£®³ĘĮæÓĆĮĖÉśŠāµÄķĄĀė£» |
| B£®½«NaOH·ÅŌŚÖ½ÕÅÉĻ³ĘĮ棻 |
| C£®NaOHŌŚÉÕ±ÖŠČܽāŗó£¬Ī“ĄäČ“¾ĶĮ¢¼“×ŖŅʵ½ČŻĮæĘæÖŠ£» |
| D£®¶ØČŻŹ±ø©ŹÓæĢ¶ČĻß |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
»Æѧ¼ĘĮæŌŚ»ÆŃ§ÖŠÕ¼ÓŠÖŲŅŖµŲĪ»£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©±ź×¼×“æöĻĀ6.72 L NH3·Ö×ÓÖŠĖłŗ¬Ō×ÓŹżÓė mL H2OĖłŗ¬Ō×ÓŹżĻąµČ”£
£Ø2£©ŅŃÖŖ16 g AŗĶ20 g BĒ”ŗĆĶźČ«·“Ӧɜ³É0£®04 mol CŗĶ31£®76 g D£¬ŌņCµÄĦ¶ūÖŹĮæĪŖ ”£
£Ø3£©°ŃV Lŗ¬ÓŠMgSO4ÓėK2SO4µÄ»ģŗĻČÜŅŗ·Ö³ÉĮ½µČ·Ż£¬Ņ»·Ż¼ÓČėŗ¬a mol NaOHµÄČÜŅŗ£¬Ē”ŗĆŹ¹Ć¾Ąė×ÓĶźČ«³ĮµķĪŖMg(OH)2£»ĮķŅ»·Ż¼ÓČėŗ¬b mol BaCl2µÄČÜŅŗ£¬Ē”ŗĆŹ¹SO42-ĶźČ«³ĮµķĪŖBaSO4£¬ŌņŌ»ģŗĻČÜŅŗÖŠ¼ŲĄė×ÓµÄĪļÖŹµÄĮæÅضČĪŖ________”££ØÓĆa”¢b”¢V±ķŹ¾£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŖ×¼Č·ÕĘĪÕ»ÆѧÓĆÓļ¼°³£ÓĆ¼ĘĮæ·½·Ø”£°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©NA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£Źż”£28gŅŅĻ©ŗĶ»·¶”Ķé£ØC4H8£©µÄ»ģŗĻĘųĢåÖŠŗ¬ÓŠ____NAøöĢ¼Ō×Ó£»·Ö×Ó×ÜŹżĪŖNAøöµÄNO2ŗĶCO2»ģŗĻĘųĢåŗ¬______ NAøöŃõŌ×ÓŹż£»1mol37ClÖŠ£¬ÖŠ×ÓŹż±ČÖŹ×ÓŹż¶ą_______ NAøö£»1L 1mol/LFe2(SO4)3ČÜŅŗÖŠŗ¬_____NAøöSO42£Ąė×Ó”£
£Ø2£©Ķ¼ÖŠA”¢B·Ö±šŹĒijĪ¢Į£µÄ½į¹¹Ź¾ŅāĶ¼£¬»Ų“šĻĀĮŠĪŹĢā£ŗ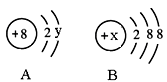
¢ŁČōA±ķŹ¾Ä³ŌŖĖŲµÄŌ×Ó£¬Ōņy£½ ”£
¢ŚČōB±ķŹ¾Ä³Ļ”ÓŠĘųĢåŌŖĖŲµÄŌ×Ó£¬ŌņøĆŌŖĖŲµÄµ„ÖŹµÄ»ÆѧŹ½ĪŖ £¬ČōBŹĒŅõĄė×ӵĽį¹¹Ź¾ŅāĶ¼£¬ŌņxµÄȔֵ·¶Ī§ŹĒ________________”£
£Ø3£©RxO42£ÖŠRµÄ»ÆŗĻ¼ŪĪŖ___________(ÓĆŗ¬x µÄŹ½×Ó±ķŹ¾)£¬µ±0.3 mol RxO42£ĶźČ«·“Ó¦£¬Éś³ÉRO2Ź±£¬×ŖŅĘ0.6 molµē×Ó£¬Ōņx£½__________”£
£Ø4£©½«7.8 gĆ¾ĀĮŗĻ½šÓė100mL Ļ”ĮņĖįĒ”ŗĆĶźČ«·“Ó¦£¬½«·“Ó¦ŗóµÄČÜŅŗ¼ÓČČÕōøÉ£¬µĆµ½ĪŽĖ®ĮņĖįŃĪ46.2 g£¬ŌņŌĮņĖįµÄĪļÖŹµÄĮæÅضČĪŖ______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Š“³öĻĀĮŠ»Æѧ·“Ó¦·½³ĢŹ½£ŗ
£Ø1£©äåŅŅĶéÓėNaOHµÄŅŅ“¼ČÜŅŗ¹²ČČ£ŗ ”£
£Ø2£©½«CO2ĶØČė±½·ÓÄĘČÜŅŗÖŠ£ŗ ”£
£Ø3£©1,3-¶”¶žĻ©µÄÓėBr2µÄ1”¢4¼Ó³É·“Ó¦£ŗ ”£
£Ø4£©ĘĻĢŃĢĒÓėŅų°±ČÜŅŗ·¢ÉśŅų¾µ·“Ó¦£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
µØ·Æ¾§ĢåŹĒĮņĖįĶµÄ½į¾§Ė®ŗĻĪļ£¬Ęä»ÆѧŹ½ĪŖCuSO4?5H2O”£ŌŚ¼ÓČČĒéæöĻĀ£¬°“ĪĀ¶Č²»Ķ¬£¬µØ·Æ¾§Ģå»įĄś¾Ņ»ĻµĮŠµÄ±ä»Æ£¬µĆµ½²»Ķ¬×é³ÉµÄ¹ĢĢ唣
£Ø1£©³ĘČ”0.1000 gŗ¬ÓŠŌÓÖŹµÄµØ·ÆŹŌѳӌ׶ŠĪĘæÖŠ£¬¼ÓČė0.1000 mol/LĒāŃõ»ÆÄĘČÜŅŗ28.00 mL£¬·“Ó¦ĶźČ«ŗ󣬹żĮæµÄĒāŃõ»ÆÄĘÓĆ0.1000 mol/LĮņĖįµĪ¶Øµ½ÖÕµć£¬ĻūŗÄĮņĖį10.08 mL£¬ŌņŹŌŃłÖŠµØ·ÆµÄÖŹĮæ·ÖŹżĪŖ___________”£
£ØŅŃÖŖ£ŗCuSO4 + 2NaOH ”ś Cu(OH)2 + Na2SO4£»ŹŌŃłÖŠµÄŌÓÖŹ²»ÓėĖį”¢¼ī·“Ó¦£©
£Ø2£©½«1.250 g“æ¾»µÄµØ·Æ¾§ĢåÖĆÓŚŪįŪöÖŠ¼ÓČČŅ»¶ĪŹ±¼ä£¬²āµĆŹ£Óą¹ĢĢåÖŹĮæĪŖ0.960 g”£Ź£Óą¹ĢĢåÖŠ½į¾§Ė®µÄÖŹĮæ·ÖŹżĪŖ__________(±£ĮōČżĪ»Š”Źż)”£
£Ø3£©½«ĪŽĖ®ĮņĖįĶ¼ÓČČÖĮ650”ęŅŌÉĻ£¬æɵƵ½ŗŚÉ«µÄŃõ»ÆĶÓėČżŃõ»ÆĮņ”¢¶žŃõ»ÆĮņŗĶŃõĘųµÄ»ģŗĻĘųĢ唣ĻÖ½«9.600 gĪŽĖ®ĮņĖįĶ³ä·Ö¼ÓČČ·Ö½āĪŖŃõ»ÆĶ£¬½«Éś³ÉµÄĘųĢåĶعż×ćĮæµÄĪüŹÕ¼Į£Ø¼īŹÆ»Ņ£©£¬ĪüŹÕ¼ĮŌöÖŲ4.416 g”£¼ĘĖć×īÖÕĪüŹÕ¼ĮÖŠĮņĖįŃĪÓėŃĒĮņĖįŃĪµÄĪļÖŹµÄĮæÖ®±Č”£
£Ø4£©ĪŽĖ®ĮņĖįĶŹÜČČ·Ö½ā³ÉŃõ»ÆĶÖ®Ē°£¬ÓŠŅ»ÖÖ»ĘÉ«ÖŠ¼ä²śĪļX³öĻÖ£¬Ęä»ÆѧŹ½æÉŅŌ±ķŹ¾ĪŖCuaOb(SO4)c£Øa”¢b”¢cĪŖÕūŹż£©”£½«X·ÅČėĖ®ÖŠ£¬ÓŠ²»Čܵĥ¶É«³ĮµķYÉś³É£Ø»ÆѧŹ½ĪŖCuSO4”¤nCu(OH)2£©£¬Ķ¬Ź±»¹ÓŠ2/3µÄĮņĖįøłČÜÓŚĖ®”£Čō¶ŌY½ųŠŠ¼ÓČČĶŃĖ®£¬½«Ź§Č„11.9%µÄÖŹĮ攣ŅŃÖŖXŗĶY¾łæÉČÜÓŚĻ”ĮņĖį”£Ķعż¼ĘĖćČ·¶ØXŗĶYµÄ»ÆѧŹ½”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com