
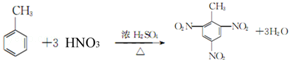
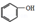
 £ØÖĘ·ÓČ©Ź÷Ö¬£©¢Ü
£ØÖĘ·ÓČ©Ź÷Ö¬£©¢Ü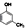 £ØÉś²śÉ±³ę¼Į£©¢ŻCH2=CH©¤CH=CH2£ØŗĻ³ÉĻš½ŗŌĮĻ£©¢ŽHCHO£Ø·ĄøƼĮ£©
£ØÉś²śÉ±³ę¼Į£©¢ŻCH2=CH©¤CH=CH2£ØŗĻ³ÉĻš½ŗŌĮĻ£©¢ŽHCHO£Ø·ĄøƼĮ£©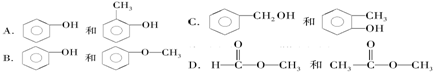
·ÖĪö £Ø1£©¢Ł¼×±½ÓėÅØĻõĖį”¢ÅØĮņĖį·¢ÉśČ”“ś·“Ӧɜ³ÉTNT£»
¢Ś60”ꏱ1£¬3-¶”¶žĻ©Óėäå1£ŗ1·¢Éś1£¬4¼Ó³É·“Ӧɜ³É1£¬4-¶žäå-2-¶”Ļ©£»
£Ø2£©£Øa£©Č©ĄąĪļÖŹŗ¬ÓŠČ©»ł£»
£Øb£©Ķ¬·ÖŅģ¹¹ĢåŹĒ·Ö×ÓŹ½ĻąĶ¬£¬½į¹¹²»Ķ¬µÄĪļÖŹ»„ĪŖĶ¬·ÖŅģ¹¹Ģ壻
£Øc£©Ķ¬ĻµĪļŹĒÖø½į¹¹ĻąĖĘ£¬×é³ÉÉĻĻą²īCH2Ō×ÓĶŵÄĪļÖŹ»„³ĘĶ¬ĻµĪļ£»
£Ø3£©øł¾ŻÓŠ»śĪļµÄ½į¹¹ŗĶ¹ŁÄÜĶÅÅŠ¶ĻĪļÖŹµÄÖÖĄą£®
½ā“š ½ā£ŗ£Ø1£©¢Ł¼×±½ÓėÅØĻõĖį”¢ÅØĮņĖį·¢ÉśČ”“ś·“Ӧɜ³ÉTNT£¬·“Ó¦ĪŖ £¬
£¬
¹Ź“š°øĪŖ£ŗ £»
£»
¢Ś60”ꏱ1£¬3-¶”¶žĻ©Óėäå1£ŗ1·¢Éś1£¬4¼Ó³É·“Ӧɜ³É1£¬4-¶žäå-2-¶”Ļ©£¬·“Ó¦·½³ĢŹ½ĪŖCH2=CH-CH=CH2+Br2”śCH2Br-CH=CH-CH2Br£¬
¹Ź“š°øĪŖ£ŗCH2=CH-CH=CH2+Br2”śCH2Br-CH=CH-CH2Br£»
£Ø2£©£Øa£©HCHOŗ¬ÓŠČ©»ł£¬ŹōÓŚČ©Ąą£¬¹Ź“š°øĪŖ£ŗ¢Ž£»
£Øb£©¢ŁCH3COOH£Øµ÷Ī¶¼Į£© ¢ŚHCOOCH3£ØŃĢ²ŻŃ¬Õō¼Į£©·Ö×ÓŹ½ĻąĶ¬£¬½į¹¹²»Ķ¬£¬»„ĪŖĶ¬·ÖŅģ¹¹Ģ壬¹Ź“š°øĪŖ£ŗ¢Ś£»
£Øc£©¢Ū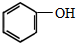 £ØÖĘ·ÓČ©Ź÷Ö¬£© ¢Ü
£ØÖĘ·ÓČ©Ź÷Ö¬£© ¢Ü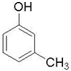 £ØÉś²śÉ±³ę¼Į£©½į¹¹ĻąĖĘ£¬×é³ÉÉĻĻą²ī1øöCH2Ō×ÓĶÅ£¬»„³ĘĶ¬ĻµĪļ£¬¹Ź“š°øĪŖ£ŗ¢Ü£»
£ØÉś²śÉ±³ę¼Į£©½į¹¹ĻąĖĘ£¬×é³ÉÉĻĻą²ī1øöCH2Ō×ÓĶÅ£¬»„³ĘĶ¬ĻµĪļ£¬¹Ź“š°øĪŖ£ŗ¢Ü£»
£Ø3£©AÖŠ¾łĪŖ“¼ĄąĪļÖŹ£¬BÖŠµŚŅ»øöĪŖ“¼Ąą£¬µŚ¶žøöĪļÖŹŹōÓŚ·ÓĄą£¬CÖŠµŚŅ»øöĪŖ“¼Ąą£¬µŚ¶žøöĪŖĆŃ£¬DÖŠ¾łĪŖõ„ĄąĪļÖŹ£¬¹ŹŹōÓŚĶ¬ĄąĪļÖŹµÄŹĒAD£¬¹Ź“š°øĪŖ£ŗAD£®
µćĘĄ ±¾Ģāæ¼²éĮĖ»Æѧ·½³ĢŹ½ŹéŠ“£¬²ąÖŲæ¼²éÓŠ»ś»Æѧ·“Ó¦·½³ĢŹ½ŹéŠ“£¬ÅŠ¶ĻÓŠ»śĪļµÄ¹ŁÄÜĶÅÓėĪļÖŹµÄĄą±š£¬±ęĪöĶ¬ĻµĪļ”¢Ķ¬·ÖŅģ¹¹ĢåµÄøÅÄī£¬Ć÷Č·ÓŠ»śĪļ½į¹¹¼°ŠŌÖŹŹĒ½āĢā¹Ų¼ü£¬×¢Ņā·“Ó¦Ģõ¼ž£¬ĢāÄæÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
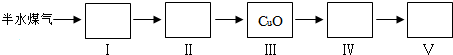
 ×Ō1913Äź¹¤ŅµŗĻ³É°±Ķ¶²śŅŌĄ“£¬ŗĻ³É°±¹¤Ņµ²»¶Ļ·¢Õ¹£¬°±ÓÖæÉŅŌ½ųŅ»²½ÖʱøĻõĖį£¬ŌŚ¹¤ŅµÉĻæɽųŠŠĮ¬ŠųÉś²ś£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
×Ō1913Äź¹¤ŅµŗĻ³É°±Ķ¶²śŅŌĄ“£¬ŗĻ³É°±¹¤Ņµ²»¶Ļ·¢Õ¹£¬°±ÓÖæÉŅŌ½ųŅ»²½ÖʱøĻõĖį£¬ŌŚ¹¤ŅµÉĻæɽųŠŠĮ¬ŠųÉś²ś£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ| t/K | 298 | 398 | 498 | ” |
| K | 4.1”Į106 | K1 | K2 | ” |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
| Na2CO3 | K2CO3 | |
| 20”ę¼īŅŗ×īøßÅØ¶Č£Ømol/L£© | 2.0 | 8.0 |
| ¼īµÄ¼Ūøń£ØŌŖ/kg£© | 1.25 | 9.80 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | SO2ŗĶNxOy¶¼ŹōÓŚĖįŠŌŃõ»ÆĪļ | |
| B£® | ±½ŹĒ×ī¼ņµ„µÄ·¼ĻćĢž | |
| C£® | ÖŲ½šŹōĄė×Óæɵ¼ÖĀµ°°×ÖŹ±äŠŌ | |
| D£® | Ęū³µĪ²ĘųµÄ“óĮæÅÅ·ÅŹĒŌģ³ÉĪķö²ĢģĘųµÄČĖĪŖŅņĖŲÖ®Ņ» |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | øĆÓŠ»śĪļµÄ»ÆѧŹ½ĪŖC3H8 | B£® | øĆÓŠ»śĪļ·Ö×ÓÖŠŅ»¶Øŗ¬ÓŠĢ¼Ģ¼Ė«¼ü | ||
| C£® | øĆÓŠ»śĪļ²»æÉÄÜŹĒŅŅĻ© | D£® | øĆÓŠ»śĪļŅ»¶Øŗ¬ÓŠŃõŌŖĖŲ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 $\frac{\underline{\;Ńõ»Æ³É\;}}{\;}$
$\frac{\underline{\;Ńõ»Æ³É\;}}{\;}$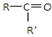 £ØR”¢R”äæɱķŹ¾Ģž»ł»ņ¹ŁÄÜĶÅ£©£¬Čō½«AÖŠĮ½»ÆŗĻĪļæ“×÷ŹĒijµ„Ļ©Ģž±»³ōŃõŃõ»ÆÉś³ÉµÄ£¬ŌņøƵ„Ļ©ĢžµÄ½į¹¹¼ņŹ½ĪŖCH3CH2CH2CH=C£ØCH3£©CH2CH3£®
£ØR”¢R”äæɱķŹ¾Ģž»ł»ņ¹ŁÄÜĶÅ£©£¬Čō½«AÖŠĮ½»ÆŗĻĪļæ“×÷ŹĒijµ„Ļ©Ģž±»³ōŃõŃõ»ÆÉś³ÉµÄ£¬ŌņøƵ„Ļ©ĢžµÄ½į¹¹¼ņŹ½ĪŖCH3CH2CH2CH=C£ØCH3£©CH2CH3£®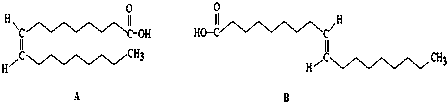
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ā±ĖŲµÄĒā»ÆĪļÖŠ·Šµć×īµĶµÄŹĒHF | |
| B£® | ŅņĪŖH2O·Ö×Ó¼äÓŠĒā¼ü£¬ĖłŅŌH2O±ČH2SĪČ¶Ø | |
| C£® | Ņ»øöXŌ×ÓµÄÖŹĮæĪŖag£¬ŌņXŌŖĖŲµÄĻą¶ŌŌ×ÓÖŹĮæĪŖaNA | |
| D£® | ČŪ·Šµć£ŗNH3£¼PCl3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com