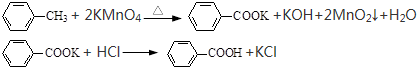
����������Al
2O
3���������������Һǿ��������ǿ�Fe
2O
3ֻ����ǿ�ᣬ�����ڼSiO
2ֻ����ǿ����������������Ʒ������ʢ��100mL H
2SO
4��Һ���ձ��У���ַ�Ӧ����ˣ�������Һ�к���Al
3+��Fe
3+����ͼ���֪������Һ�м���10mol?L
-1��NaOH��Һ��û�г������ɣ�˵��������ʣ�࣬��������NaOH��Һ�������ӱ�����������������������ӱ��Ϊ���������������������������ƹ�35mLʱ�����������ʱ��Һ������Ϊ�����ƣ������������غ���������Ƶ����ʵ������ٸ���������غ����ԭ������Һ��n��H
2SO
4�����ٸ���c=
���㣻
��35mL��45mL�����10mL����������ȫ�ܽ�����������������Ӧ��Al��OH��
3+OH
-=AlO
2-+2H
2O�����ʣ�����Ϊ��������������n=cV����ý����ĵ�n��NaOH�����ٸ��ݷ���ʽ�����������������ʵ�����������Ԫ���غ����n��Al
2O
3��������m=nM������������������������������������㣻
������Ԫ���غ������Һ��n��Al
3+��������������������ȫ�������ĵ��������Ƶ����ʵ���������n=cV���������ӡ���������ȫ���ĵ�n��NaOH������������n��Fe
3+����������Ԫ���غ����n��Fe
2O
3��������m=nM������������������������������������㣮
���
�⣺��1��B��ʱ�������ӱ�����������������������ӱ����������������B��ʱ�������ijɷ�����������������������
�ʴ�Ϊ����������������������
��2����ͼ���֪������Һ�м���10mol?L
-1��NaOH��Һ��û�г������ɣ�˵��������ʣ�࣬OA�����������������Ʒ�Ӧ�����ӷ���ʽΪ��H
++OH
-=H
2O��BC��������������ȫ�ܽ�����������������Ӧ�����ӷ���ʽΪ��Al��OH��
3+OH
-=AlO
2-+2H
2O��
�ʴ�Ϊ��H
++OH
-=H
2O��Al��OH��
3+OH
-=AlO
2-+2H
2O��
��3��������������35mLʱ�����������ʱ��Һ����Ϊ�����ƣ�����NaԪ���غ���n��Na
2SO
4��=
n��NaOH��=
��0.035L��10mol?L
-1=0.175mol������������غ㣬���У�n��H
2SO
4��=0.175mol��
����ԭ������Һ��c��H
2SO
4��=
=1.75mol/L��
�ʴ�Ϊ��1.75��
��4����35mL��45mL�����10mL����������ȫ�ܽ������������ý����ĵ�n��NaOH��=0.01L��10mol?L
-1=0.1mol�����ݷ���ʽAl��OH��
3+NaOH=NaAlO
2+2H
2O��֪��n[Al��OH��
3]=0.1mol������Һ��n��Al
3+��=0.1mol������AlԪ���غ��֪n��Al
2O
3��=
��0.1mol=0.05mol����m��Al
2O
3��=0.05mol��102g/mol=5.1g��
��������ȫ���������������Ƶ����ʵ���Ϊ0.1mol��3=0.3mol�������ӡ���������ȫ�������ĵ�����������Һ�����Ϊ35mL-2.3mL=32.7mL���ʸý�����n��NaOH��=0.0327L��10mol/L=0.327mol������������ȫ�������ĵ��������Ƶ����ʵ���Ϊ0.327mol-0.3mol=0.027mol��
����������ȫ�������ĵ��������Ƶ����ΪV=
=
=0.0027L=2.7ml����n��Fe
3+��=
=0.009mol����m��Fe
2O
3��=0.009mol��
��160g/mol=0.72g��m��SiO
2��=6-0.72-5.1=0.18g��
�������и���ɳɷֵ���������Ϊ��Al
2O
3 Ϊ
��100%=85%��Fe
2O
3 Ϊ
��100%=12%��
�ʴ�Ϊ��85%��12%��

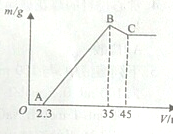 ȷ��ȡ6g��������Ʒ����Al2O3��Fe2O3��SiO2������ʢ��100mLijŨ��������Һ���ձ��У���ַ�Ӧ����ˣ�����Һ�м���10mol?L-1��NaOH��Һ�������ij�������m������NaOH��Һ�����V�Ĺ�ϵ��ͼ��ʾ����ش��������⣺
ȷ��ȡ6g��������Ʒ����Al2O3��Fe2O3��SiO2������ʢ��100mLijŨ��������Һ���ձ��У���ַ�Ӧ����ˣ�����Һ�м���10mol?L-1��NaOH��Һ�������ij�������m������NaOH��Һ�����V�Ĺ�ϵ��ͼ��ʾ����ش��������⣺

 ����������ϵ�д�
����������ϵ�д�