
| �ζ����� | ������Һ �����mL�� | ������� | |
| �ζ�ǰ�Ŀ̶ȣ�mL�� | �ζ���Ŀ̶ȣ�mL�� | ||
| ��һ�� | 10 | 0.5 | 20.6 |
| �ڶ��� | 10 | 4.2 | 24.1 |
���� ��1���׳����ҩƷ��������ڲ��������ϣ��磺С�ձ����������������
��2������к͵ζ�ʱ�����÷�̪�������ָʾ����ʯ���ɫ��Χ�����������ԣ���һ�㲻��ʯ����ָʾ����
��3���ζ�ʱ�����ֿ��Ƶζ��ܣ�����ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�ı仯��Ϊ���ڹ۲���Һ��ɫ�仯������ƿ�µ�һ�Ű�ֽ��
��4���Ⱦ�����ʵ���������Ҫ��Һ��������پ�c�����⣩=c$\frac{c��������V������}{V�����⣩}$����Ũ�ȣ�8.6g��Ʒ������Һ���Ϊ500mL����10mL��ҺŨ������500mL��Һ������NaOH�����������㴿�ȣ�
��5������c�����⣩=c$\frac{c��������V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��� �⣺��1���׳����ҩƷ��������ڲ��������ϣ��磺С�ձ������������������ֹ�������̣����ռ��׳��⣬����Ӧ����С�ձ��г�����
��ѡC��
��2������к͵ζ�ʱ�����÷�̪�������ָʾ����ʯ���ɫ��Χ�����������ԣ���һ�㲻��ʯ����ָʾ����
��ѡA��
��3���ζ�ʱ���ζ�����������Ӧ��ע����ƿ����Һ����ɫ�仯����ʢ�Ŵ�����Һ����ƿ�·���һ�Ű�ֽ�������ǹ۲���ƿ����Һ��ɫ�ı仯���ԣ�����ʵ����
�ʴ�Ϊ����ƿ����Һ����ɫ�仯�����ڹ۲���ƿ��Һ����ɫ�ı仯����С�ζ���
��4���������ĵı�Һ������ֱ�Ϊ��20.10mL��19.90mL�����εζ����ݶ�����Ч�ģ��������ı�Һƽ�����Ϊ20.00mL��
����c�����⣩=c$\frac{c��������V������}{V�����⣩}$=$\frac{0.2000mol•L{\;}^{-1}��0.02L}{0.01L}$=0.4000mol•L-1��
��Ʒ�к����ռ������Ϊ��m���ռ=c•V•M=0.4000mol•L-1��0.5L��40g/mol=8.0g���ռ����������Ϊ����=$\frac{8.0g}{8.6g}$��100%=93.02%��
�ʴ�Ϊ��0.4000��93.02%��
��5���ٵζ�ǰ��ʽ�ζ��ܼ��촦�����ݣ��ζ��������Һ�����V������ƫ�ߣ�����c�����⣩=c$\frac{c��������V������}{V�����⣩}$��������֪c�����⣩ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
�ڶ�ȡ����Һ���ʱ���ζ�ǰ���ӣ��ζ��յ�ʱƽ�ӣ����V������ƫ�ͣ�����c�����⣩=c$\frac{c��������V������}{V�����⣩}$��������֪c�����⣩ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
���� ���⿼��������к͵ζ��еIJ���Ҫ�㡢�еζ��ļ����Լ�����������Ŀ�ѶȲ���


 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
���������ν�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ��һ�ϵ�һ���¿���ѧ���������棩 ���ͣ�ʵ����
ʵ������Ҫ450 mL0.2 mol��L��1�����ᣬ������������Ϊ98%���ܶ�Ϊ1.84 g��cm��3��Ũ���������ơ���ش��������⣺
(1)������������˲��������ձ�����Ͳ���������õ��IJ��������ǣ�___________
(2)������Һʱ��һ����Է�Ϊ���¼������裺����ȡ �ڼ��� ���ܽ� �ܵ�תҡ�� ��ת�� ��ϴ�� �߶��� ����ȴ������ȷ�IJ���˳��Ϊ__________________��
(3)�����㣬��Ũ��������Ϊ ��
(4)������ƿ��ʹ�÷����У����в�������ȷ���ǣ����ţ�________��
A��������ƿ��ת����ҺʱӦ�ò��������� |
B������ƿ������ˮϴ�������ô�����Һ��ϴ |
C��������Һʱ����������ǹ��壬�ѳƺõ�������ֽ��С�ĵ�������ƿ�У�������������ˮ���ӽ�����2��3cm�����õιܼ�����ˮ������ |
D��������Һʱ�����������Һ�壬����Ͳ��ȡ������ֱ���ز���������������ƿ�У�������������ˮ������ |
E���Ǻ�ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ������ת���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������ͭ��ʹп��ϡ����ķ�Ӧ���ʼӿ죬˵��Cu2+���д����� | |
| B�� | ����HA��HB�ĵ��볣������Դ�С����ֱ���ж�������Һ������ǿ�� | |
| C�� | ������2S2O��g���T3S��s��+SO2��g�����Է����У����ƶϸ÷�ӦΪ���ȷ�Ӧ | |
| D�� | ��Ũ�����Ũ�����Ϻ�����������Ƭ����Ƭ���ۻ���Ч��һ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.01 mol•L-1HA����Һ��c��H+��=1��10-4mol•L-1 | |
| B�� | pH=3��HA��Һ��pH=11��NaOH��Һ�������Ϻ�������Һ��c��Na+����c��A-����c��OH-����c��H+�� | |
| C�� | Ũ�Ⱦ�Ϊ0.1 mol•L-1��HA��Һ��NaA��Һ�������Ϻ�������Һ�����ԣ��� c��OH-��-c��H+����c��HA��-c��A-�� | |
| D�� | pH=3��HA��Һ��pH=11��NaOH��Һ�������1��10��Ϻ�������Һ�� c��OH-��+c��A-���Tc��H+��+c��Na+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 CH3COOHΪ���������ᣬ�ڹ�ҵ�������������й㷺��Ӧ�ã�
CH3COOHΪ���������ᣬ�ڹ�ҵ�������������й㷺��Ӧ�ã��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ͬ�¶��£��������Ȼ�������ֱ������ͬ����Ģ�����ˮ ��0.1 mol/L�����0.1 mol/L�Ȼ�þ��Һ ��0.1 mol/L��������Һ�У�Ag+Ũ�ȣ��٣���=�ڣ��� | |
| B�� | �ں���BaSO4��������Һ�м���Na2SO4���壬c��Ba2+������ | |
| C�� | ��Mg��OH��2����Һ�еμ�FeCl3��Һ��������Ϊ���ɫ��˵���ܽ��Mg��OH��2��Fe��OH��3 | |
| D�� | ��֪I3-?I2+I-����ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
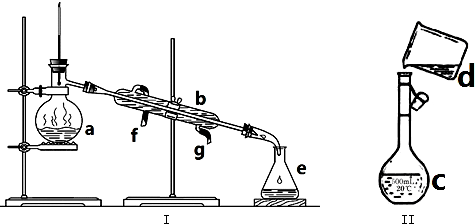
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | b��c | B�� | a+b��2c | C�� | a+b��c | D�� | a+b��2c |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com