
��14�֣�A��I�����л���������ת����ϵ��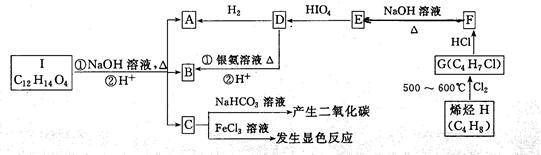
��֪��R1CHOHCHOHR2 + HIO4����R1CHO+ R2CHO+HIO3 +H2O����֪C�ı����ϵ�һ�ȴ�����2�֡���ش��������⣺
��1��д���������ʵĽṹ��ʽ��A ��I �� C�еĹ��������� ����˴Ź�������ͼ���� �塣
��2����H����G���л���Ӧ����Ϊ ��
��3��д��ʵ������ת����Ӧ�Ļ�ѧ����ʽD��B , F��E��Ӧ�Ļ�ѧ����ʽ ��
��4��������C��ͬ���칹�����࣬д�������������������з�����ͬ���칹��Ľṹ��ʽ
�����ڶ�λ��ȡ���� ���ܷ���������Ӧ ����NaOH��Һ�пɷ���ˮ�ⷴӦ
��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��1��������д��A��B�Ļ�ѧʽ��____________________��____________________��
��2��������д��G��C�ĵ���ʽ��_____________________��___________________��
��3����д�����±仯�Ļ�ѧ����ʽ��
D��E__________________________________________________________��
C+F��I+O2_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ����5��ģ����������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
��14�֣�A��I�����л���������ת����ϵ��

��֪��R1CHOHCHOHR2 + HIO4����R1CHO+ R2CHO+HIO3 +H2O����֪C�ı����ϵ�һ�ȴ�����2�֡���ش��������⣺
��1��д���������ʵĽṹ��ʽ��A ��I �� C�еĹ��������� ����˴Ź�������ͼ���� �塣
��2����H����G���л���Ӧ����Ϊ ��
��3��д��ʵ������ת����Ӧ�Ļ�ѧ����ʽD��B , F��E��Ӧ�Ļ�ѧ����ʽ ��
��4��������C��ͬ���칹�����࣬д�������������������з�����ͬ���칹��Ľṹ��ʽ
�� ���ڶ�λ��ȡ���� �� �ܷ���������Ӧ ����NaOH��Һ�пɷ���ˮ�ⷴӦ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���Ĵ�ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪ ������A��B��C��D��E��F�����л���������ת��������A�ķ���ʽΪC4H8O3 �������ж���ȷ����
������A��B��C��D��E��F�����л���������ת��������A�ķ���ʽΪC4H8O3 �������ж���ȷ����

A���л���A�ṹ���������� B����Ӧ������������Ӧ
C���л���B��D�����ʽ��ͬ D���л���E�ǽ������Ƽݵ���Ҫ��֤
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)������д��A��B�Ļ�ѧʽ��____________��____________��
(2)������д��G��C�ĵ���ʽ��___________��__________��
(3)��д�����±仯�Ļ�ѧ����ʽ��___________________
D![]() E________________________________________________��
E________________________________________________��
C+F_________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com