
����Ŀ��H2RO3��һ�ֶ�Ԫ�ᣬ��������1L1mol��L-1Na2RO3��Һ����RO2���壬��Һ��pH��RO2��������ʵ����ı仯��ͼ��ʾ������˵����ȷ����
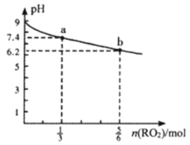
A.a����Һ��2c(Na+)=3c(RO32-)
B.��b����Һ�м�ˮ��ʹ��Һ��pH��6.2���ߵ�7.4
C.�����£�NaHRO3��Һ��c(HRO3-)>c(RO32-)>c(H2RO3)
D.������RO2����Һ������ʱ��c(Na+)=c(RO32-)+c(HRO3-)
���𰸡�C
��������
��ͼ��֪��Na2RO3��Һ�ʼ��ԣ�˵��H2RO3��һ�ֶ�Ԫ���ᣬ��1L1mol��L-1Na2RO3��Һ����RO2���壬Na2RO3��Һ��RO2���巴Ӧ������ʽ��NaHRO3����Ӧ�Ļ�ѧ����ʽΪNa2RO3+H2O+RO2=2NaHRO3��a������![]() mol RO2���壬��Һ�к���
mol RO2���壬��Һ�к���![]() mol Na2RO3��
mol Na2RO3��![]() mol NaHRO3����Һ�ʼ��ԣ�b������
mol NaHRO3����Һ�ʼ��ԣ�b������![]() mol RO2���壬��Һ�к���
mol RO2���壬��Һ�к���![]() mol Na2RO3��
mol Na2RO3��![]() mol NaHRO3����Һ�����ԡ�
mol NaHRO3����Һ�����ԡ�
A. a������![]() mol RO2���壬��Һ��Na+�����ʵ���Ϊ2mol��RO32-�����ʵ���Ϊ
mol RO2���壬��Һ��Na+�����ʵ���Ϊ2mol��RO32-�����ʵ���Ϊ![]() mol��RO32-��������Һ��ˮ�⣬��2c(Na+)>3c(RO32-)����A����
mol��RO32-��������Һ��ˮ�⣬��2c(Na+)>3c(RO32-)����A����
B. b�㵼����Һ�����ԣ�����Һ��ˮϡ�ͣ�������Ũ����С��pHֻ�����ӽ�7�����ᳬ��7����B����
C. b������![]() mol RO2���壬��Һ�к���
mol RO2���壬��Һ�к���![]() mol Na2RO3��
mol Na2RO3��![]() mol NaHRO3����Һ������˵��NaHRO3��Һ��HRO3-����̶ȴ���ˮ��̶ȣ���c(
mol NaHRO3����Һ������˵��NaHRO3��Һ��HRO3-����̶ȴ���ˮ��̶ȣ���c(![]() )>c(
)>c(![]() )>c(H2RO3)����C��ȷ��
)>c(H2RO3)����C��ȷ��
D. ������RO2����Һ������ʱ����Һ��c(H+)=c(OH-)�����ݵ���غ�c(H+)+c(Na+)=2c(RO32-)+c(HRO3-)+c(OH-)�ɵ�c(Na+)=2c(RO32-)+c(HRO3-)����D����
��ѡC��


 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ҵ��ˮ�н����±������е�5�֣�
������ |
|
������ |
|
ijͬѧ��̽����ˮ����ɣ�����������ʵ�飺
��![]() ȡ��ˮ���������������ᣬ�ް�ɫ������������������ʹ����ʯ��ˮ����ǵ���ɫ��ζ����
ȡ��ˮ���������������ᣬ�ް�ɫ������������������ʹ����ʯ��ˮ����ǵ���ɫ��ζ����
��![]() ��������õ���Һ�м���
��������õ���Һ�м���![]() ��Һ���а�ɫ�������ɣ�
��Һ���а�ɫ�������ɣ�
�����ƶϲ���ȷ����![]()
A.��Һ��һ�����е�������![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]()
B.���м�������������ɫ��������ӷ���ʽ��![]()
C.ԭ��Һ�е�![]() ��
��![]() ��
��![]() ���������ȷ��
���������ȷ��
D.���в�����ɫ���������ӷ���ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���˿�ѧ�Һ������Ŷ��з�����ֽ����� (��ͼ)������һ���п��һ��ƶ������̵ij��������ʹ��ӡˢ��ѹ�㼼�����һ�ſ�����ü���С������ֽ������Ƚ�Ϊ0.5���ף��������������Ͳü���ֽ�ڵ�������������ˮ������п��ɵ��Һ������ܷ�ӦʽΪ�� Zn+2MnO2+H2O=ZnO+2MnO(OH)������˵����ȷ����

A.�õ�ص���������Ϊп
B.�õ�ط�Ӧ�ж������̷�����������Ӧ
C.��ص�������ӦʽΪ2MnO2 +2H2O+2e-= 2MnO(OH)+2OH-
D.����0.1molп�ܽ�ʱ���������Һ�ĵ�����Ϊ1.204��1023
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о�С����ϳ�Ⱦ��X��ҽҩ�м���Y��
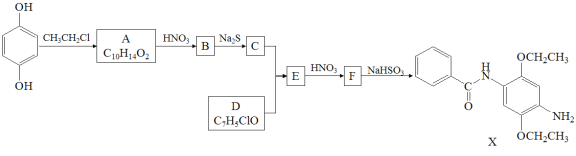
��֪��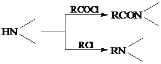 ��
��![]()
��ش�
(1)����˵����ȷ����____________��
A��������A����FeCl3��Һ������ɫ��Ӧ B��������C����������
C��������F�ܷ����ӳɡ�ȡ������ԭ��Ӧ D��X�ķ���ʽ��C17H22N2O3
(2)������B�Ľṹ��ʽ��___________________________________________________��
(3)д��C+D��E�Ļ�ѧ����ʽ_____________________________________________________��
(4)д��������A(C10H14O2)ͬʱ��������������ͬ���칹��Ľṹ��ʽ_____________________________��
1H��NMR��IR���������ٷ�������4�ֻ�ѧ������ͬ����ԭ�ӣ��ڷ����к��б�����������(��OCH3)��û���ǻ���������(��O��O��)��
(5)�����CH2=CH2��![]() Ϊԭ���Ʊ�Y(
Ϊԭ���Ʊ�Y(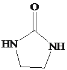 )�ĺϳ�·��(������ͼ��ʾ�����Լ���ѡ)
)�ĺϳ�·��(������ͼ��ʾ�����Լ���ѡ)
_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼���γɻ�������������Ԫ�أ��䵥�ʼ��仯���������������������Ҫ��Դ���ʡ���ش��������⣺
(1)�л���M����̫������տ�ת����N��ת���������£���H��+88.6 kJ/mol��M��N��ȣ����ȶ�����________��
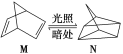
(2)��֪CH3OH(l)��ȼ����Ϊ-726.5 kJ��mol��1��CH3OH(l)��1/2O2(g)=CO2(g)��2H2(g) ��H����a kJ��mol��1����a________726.5(����������������������)��
(3)ʹCl2��H2O(g)ͨ�����ȵ�̿�㣬����HCl��CO2������1 mol Cl2���뷴Ӧʱ�ͷų�145 kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��_______________________________��
(4) ��֪��Fe2O3(s)+3C(ʯī)=2Fe(s)+3CO(g) ��H=+489.0 kJ mol-1��CO(g)+l/2O2(g)=CO2(g)��H=-283. 0kJ mol-1��C(ʯī)+O2(g)=CO2(g)��H=-393.5kJ mol-1����4Fe (s)+3O2(g) =2Fe2O3(s)��H=____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��(N2H4)��ǿ�������������ⳣ�������ȼ��,������ֹ��������ѺϽ���ϡ���ش���������:
��1��N2H4��H2O2�����Ԫ���е�һ������������______��
��2����Ԫ�ػ�̬ԭ�ӵĺ�������Ų�ʽΪ_____________________��
��3��1 mol N2H4�������������Լ�����ĿΪ___________��
��4��H2O2�����ֽ�ΪH2O,H2O�Ŀռ乹��Ϊ_______,������ԭ�ӵ��ӻ��������Ϊ_________��
��5��H2S��H2O2����Ҫ�����������±���ʾ:
���� | �۵�/K | �е�/K | ˮ���ܽ��(��״��) |
H2S | 187 | 202 | ÿ��ˮ���ܽ�2.6 L |
H2O2 | 272 | 423 | ������Ȼ��� |
��������۷е���ܽ�Ȳ������Ҫԭ��ֱ���_______________��________________��
��6������þ�͵���������������زյ������β���,������:����þ_______(����ڡ���С�ڡ�)�����ơ�
��7������������ľ�����ͼ��ʾ�����ھ��������ԭ�ӵ���λ��Ϊ______���������߳�Ϊa cm,��������������ܶ���________________g��cm-3��(ֻҪ���г���ʽ,�谢���ӵ�������ֵΪNA)
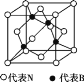
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ����(�� ��)
A.����ʱ��ij��Һ����ˮ���������c(H��)��c(OH��)�ij˻�Ϊ1��10��24������Һ��һ�����Դ�������K����Na����AlO2-��SO42-
B.����c(H��)�͵����������ʹ����У��ֱ��������ͬ��пƬ����ͬ״���£�����ᷴӦ�����������������
C.��֪H2CO3 �� Ka1= 4.30��10-7��Ka2= 5.61��10-11��HClO�� Ka = 3.0��10-8����ӦCO2+H2O+2NaClO=Na2CO3+2HClO�ܹ�����
D.��c(H��)������ʹ��ᣬ��ˮϡ��100����c(H��)ǰ�ߴ��ں���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ƣ�Na2S2O5����һ�ֳ��õĿ���������
ij�о�С��Խ��������ƽ��������о���
��1��������ͼװ�ã�ʵ��ǰ�ѳ���װ���ڵĿ�������ȡNa2S2O5��
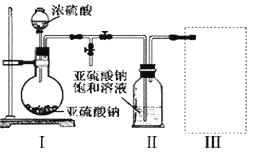
װ�â�����Na2S2O5�����������Ļ�ѧ��Ӧ����ʽΪ��Na2SO3+ SO2= Na2S2O5��
��װ�â��в�������Ļ�ѧ����ʽΪ_____________________________��
��Ҫ��װ�â��л���������ľ��壬�ɲ�ȡ�ķ��뷽����______________��
��װ�â����ڴ���β������ѡ�õ������װ�ã��г���������ȥ��Ϊ______������ţ���
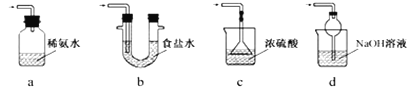
��2�����������ϣ�Na2S2O5����ˮ������NaHSO3��
��NaHSO3��Һ�����ԡ����û�ѧ����ͱ�Ҫ�����ֽ���ԭ��________________________________��
֤���ý��ۿɲ��õ�ʵ�鷽����_______________������ţ���
a���ⶨ��Һ��pH
b������Ba(OH)2��Һ
c����������
d������Ʒ����Һ
e������ɫʯ����ֽ���
�ڼ���Na2S2O5�����ڿ������ѱ�������ʵ�鷽����__________________��
��3�����������ƣ�Na2S2O5�������������¿ɽ���ҵ��ˮ�е�Cr2O72����ԭΪCr3+��
��д���÷�Ӧ�����ӷ���ʽ_______________________��
��������Cr2O72��Ũ��Ϊ1��10-3mol/L�Ĺ�ҵ��ˮ1L������Na2S2O5����_________mg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����̼����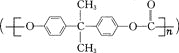 ��һ�ָ����Ե���������֬���㷺����������ҵ�������DZ�������������������������ɫ��ѧԭ��X
��һ�ָ����Ե���������֬���㷺����������ҵ�������DZ�������������������������ɫ��ѧԭ��X![]() ����һԭ��Y��Ӧ�Ƶá�����˵������ȷ���ǣ� ��
����һԭ��Y��Ӧ�Ƶá�����˵������ȷ���ǣ� ��
A.���Ʊ���ӦΪ���۷�Ӧ
B.Y�ķ���ʽΪC15H16O2
C.��ͳ�ĺϳɷ���ԭ��Xһ��ѡ��![]() ����ϳɲ����б��д���HCl�������
����ϳɲ����б��д���HCl�������
D.����ԭ��XѡΪCO2���ڴ���������ʵ������ԭ��������100%
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com