
(14·Ö)øßĆĢĖį¼ŲĖ׳ĘPP·Ū£¬ĪŖĒæŃõ»Æ¼Į£¬Óöµ½ÓŠ»śĪļ¾Ķ·Å³ö»īŠŌŃõ”£ÕāÖÖŃõӊɱƚĻø¾śµÄ×÷ÓĆ£¬ĒŅɱ¾śÄÜĮ¦¼«Ēæ”£Ä³Ń§ÉśÓūŌŚŹµŃéŹŅÅäÖĘ1 L 0.06 mol/L KMnO4Ļ”ČÜŅŗ£¬ÓĆĄ“ĒåĻ“ÉĖæŚ”£
(1)ŹµŃé¹ż³ĢÖŠŠčÓĆĶŠÅĢĢģĘ½³ĘČ”KMnO4¾§ĢåµÄÖŹĮæĪŖ__________g”£
(2)Ęä²Ł×÷²½ÖčČēĻĀĶ¼ĖłŹ¾£¬ŌņÓŅĶ¼ĖłŹ¾²Ł×÷Ó¦ŌŚĻĀĶ¼ÖŠ__________(ĢīŃ”Ļī×ÖÄø)Ö®¼ä”£
A£®¢ŚÓė¢Ū””””B£®¢ŁÓė¢Ś””””C£®¢ÜÓė¢Ż
(3) ÅäÖĘ¹ż³ĢÖŠŠčŅŖÓƵ½µÄŅĒĘ÷ÓŠ:ĶŠÅĢĢģĘ½”¢Ņ©³×”¢ÉÕ±”¢ ”¢ ”¢
(4)ČōøĆĶ¬Ń§ŌŚÅäÖĘČÜŅŗŹ±£¬½ųŠŠĮĖČēĻĀ²Ł×÷£¬ĘäÖŠŹ¹ĖłÅäČÜŅŗÅضČĘ«µĶµÄ²Ł×÷
ÓŠ_______________(ĢīŃ”Ļī×ÖÄø)”£
A£®³ĘĮæKMnO4¾§ĢåŹ±£¬ÖøÕėĘ«ĻņÓŅ±ß
B£®½«KMnO4¾§Ģå·ÅŌŚÉÕ±ÖŠČܽā£¬ĄäČ“ŗó£¬×ŖŅĘÖĮŗ¬ÓŠÉŁĮæÕōĮóĖ®µÄČŻĮæĘæÖŠ
C£®¶ØČŻŹ±£¬ŃöŹÓæĢ¶ČĻß
D£®Õńµ“Ņ”ŌČŗ󣬷¢ĻÖČÜŅŗŅŗĆęµĶÓŚæĢ¶ČĻߣ¬ŌŁµĪ¼ÓÉŁĮæÕōĮóĖ®ÖĮæĢ¶ČĻß“¦

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģɽ¶«Ź”¼ĆÄžŹŠćōĖ®Ņ»ÖŠøßČżÉĻŃ§ĘŚĘŚÄ©Ä£Äā»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
(14·Ö)
¢ń£®£Ø1£©·ÖĪöĻĀ±ķÖŠø÷ĻīµÄÅŲ¼¹ęĀÉ£¬°““Ė¹ęĀÉÅŲ¼µŚ22ĻīÓ¦ĪŖ ”£
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| C2H4 | C2H6 | C2H6O | C2H4O2 | C3H6 | C3H8 | C3H8O | C3H6O2 | C4H8 | C4H10 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģɽ¶«Ź”»øĢصŚ¶žÖŠŃ§øßČż11ŌĀÄ£æé¼ģ²ā»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
(14·Ö)øßĆĢĖį¼ŲĖ׳ĘPP·Ū£¬ĪŖĒæŃõ»Æ¼Į£¬Óöµ½ÓŠ»śĪļ¾Ķ·Å³ö»īŠŌŃõ”£ÕāÖÖŃõӊɱƚĻø¾śµÄ×÷ÓĆ£¬ĒŅɱ¾śÄÜĮ¦¼«Ēæ”£Ä³Ń§ÉśÓūŌŚŹµŃéŹŅÅäÖĘ1 L 0.06 mol/L KMnO4Ļ”ČÜŅŗ£¬ÓĆĄ“ĒåĻ“ÉĖæŚ”£
(1)ŹµŃé¹ż³ĢÖŠŠčÓĆĶŠÅĢĢģĘ½³ĘČ”KMnO4¾§ĢåµÄÖŹĮæĪŖ__________g”£
(2)Ęä²Ł×÷²½ÖčČēĻĀĶ¼ĖłŹ¾£¬ŌņÓŅĶ¼ĖłŹ¾²Ł×÷Ó¦ŌŚĻĀĶ¼ÖŠ__________(ĢīŃ”Ļī×ÖÄø)Ö®¼ä”£
A£®¢ŚÓė¢Ū””””B£®¢ŁÓė¢Ś””””C£®¢ÜÓė¢Ż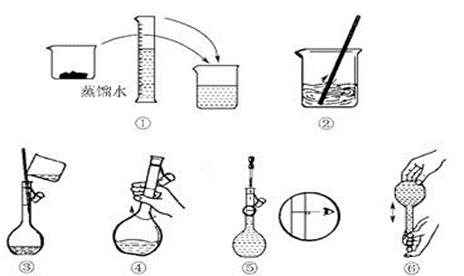
(3) ÅäÖĘ¹ż³ĢÖŠŠčŅŖÓƵ½µÄŅĒĘ÷ÓŠ:ĶŠÅĢĢģĘ½”¢Ņ©³×”¢ÉÕ±”¢ ”¢ ”¢
(4)ČōøĆĶ¬Ń§ŌŚÅäÖĘČÜŅŗŹ±£¬½ųŠŠĮĖČēĻĀ²Ł×÷£¬ĘäÖŠŹ¹ĖłÅäČÜŅŗÅضČĘ«µĶµÄ²Ł×÷
ÓŠ_______________(ĢīŃ”Ļī×ÖÄø)”£
A£®³ĘĮæKMnO4¾§ĢåŹ±£¬ÖøÕėĘ«ĻņÓŅ±ß
B£®½«KMnO4¾§Ģå·ÅŌŚÉÕ±ÖŠČܽā£¬ĄäČ“ŗó£¬×ŖŅĘÖĮŗ¬ÓŠÉŁĮæÕōĮóĖ®µÄČŻĮæĘæÖŠ
C£®¶ØČŻŹ±£¬ŃöŹÓæĢ¶ČĻß
D£®Õńµ“Ņ”ŌČŗ󣬷¢ĻÖČÜŅŗŅŗĆęµĶÓŚæĢ¶ČĻߣ¬ŌŁµĪ¼ÓÉŁĮæÕōĮóĖ®ÖĮæĢ¶ČĻß“¦
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģ½ĖÕŹ”Ńļ֯֊ѧøßČż4ŌĀĖ«ÖÜĮ·Ļ°»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
(14·Ö) ²ŻĖįŃĒĢśÓĆ×÷·ÖĪöŹŌ¼Į¼°ĻŌÓ°¼ĮµČ£¬ĘäÖʱøĮ÷³ĢČēĻĀ£ŗ
¢ÅÅäÖĘ(NH4)2Fe(SO4)2 6H2OČÜŅŗŹ±£¬Šč¼ÓÉŁĮæĻ”ĮņĖį£¬ÄæµÄŹĒ ”£
6H2OČÜŅŗŹ±£¬Šč¼ÓÉŁĮæĻ”ĮņĖį£¬ÄæµÄŹĒ ”£
¢Ę½«ÖĘµĆµÄ²śĘ·ŌŚė²ĘųĘų·ÕÖŠ½ųŠŠČČÖŲ·ÖĪö£¬½į¹ūČēÓŅĶ¼£ØTG%±ķŹ¾²ŠĮō¹ĢĢåÖŹĮæÕ¼Ōѳʷ×ÜÖŹĮæµÄ°Ł·ÖŹż£©”£
¢ŁŌņC“¦Ź±²ŠĮōĪļµÄ»ÆѧŹ½ĪŖ ”£
¢ŚÓŠŃŠ¾æѧÕßŌŚŹµŃé¹ż³ĢÖŠÓĆĘųĻąÉ«Ę×»¹¼ģ³öH2£¬×īÖÕ²śĪļÖŠŅ²ÓŠĪ¢ĮæµÄ“ÅŠŌĪļÖŹÉś³É£¬ĒėÄć²ĀĻėÓĆŅ»øö·½³ĢŹ½½āŹĶÕāÖÖŹĀŹµ£ŗ ”£
¢ŪĻÖČ”ŌŚÕęæÕÖŠ146”ęĶŃĖ®ŗóµÄFeC2O41.44g·ÅŌŚÄ³ÕęæÕµÄĆܱÕČŻĘ÷ÖŠ£¬ŌŁ³äČė0.04molCO”£¼ÓČČÖĮ1100”ę£¬ĘäÖŠFeO(s)+CO(g) Fe(s)+CO2(g)·“Ó¦Ę½ŗā³£ŹżK=0.4£¬ŌņøĆ·“Ó¦“ļĘ½ŗāŹ±£¬FeOµÄ×Ŗ»ÆĀŹĪŖ¶ąÉŁ£æ ”£
Fe(s)+CO2(g)·“Ó¦Ę½ŗā³£ŹżK=0.4£¬ŌņøĆ·“Ó¦“ļĘ½ŗāŹ±£¬FeOµÄ×Ŗ»ÆĀŹĪŖ¶ąÉŁ£æ ”£
£Ø3£©½šŹōĆ¾ŌŚ¹śĆńÉś²śÖŠÓŠÖŲŅŖ×÷ÓĆ£¬³£ŅŌMgCl2ĪŖŌĮĻ»ńČ”£¬ĪĀ¶ČŗĶŃ¹ĒæP(HCl)g¶ŌMgCl2”¤6H2O¾§ĢåČČ·Ö½ā²śĪļµÄÓ°ĻģČēĶ¼ĖłŹ¾”£½įŗĻÓŅĶ¼Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ŁŠ“³öP(HCl)g = 0.25MPa£¬ĪĀ¶Č“Ó300”ęÉżøßµ½550”ꏱ·“Ó¦µÄ»Æѧ·½³ĢŹ½ £»
¢ŚŹµ¼ŹÉś²śÖŠ£¬½«MgCl2”¤6H2O¾§Ģå¼ÓČȵ½600”ęµÄ¹ż³ĢÖŠ¼øŗõµĆ²»µ½ĪŽĖ®MgCl2£¬ĘäŌŅņŹĒ £»ČōŅŖµĆµ½ĪŽĖ®MgCl2Šė²ÉČ”µÄ“ėŹ©ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012Ń§ÄźÉ½¶«Ź”øßČż11ŌĀÄ£æé¼ģ²ā»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
(14·Ö)øßĆĢĖį¼ŲĖ׳ĘPP·Ū£¬ĪŖĒæŃõ»Æ¼Į£¬Óöµ½ÓŠ»śĪļ¾Ķ·Å³ö»īŠŌŃõ”£ÕāÖÖŃõӊɱƚĻø¾śµÄ×÷ÓĆ£¬ĒŅɱ¾śÄÜĮ¦¼«Ēæ”£Ä³Ń§ÉśÓūŌŚŹµŃéŹŅÅäÖĘ1 L 0.06 mol/L KMnO4Ļ”ČÜŅŗ£¬ÓĆĄ“ĒåĻ“ÉĖæŚ”£
(1)ŹµŃé¹ż³ĢÖŠŠčÓĆĶŠÅĢĢģĘ½³ĘČ”KMnO4¾§ĢåµÄÖŹĮæĪŖ__________g”£
(2)Ęä²Ł×÷²½ÖčČēĻĀĶ¼ĖłŹ¾£¬ŌņÓŅĶ¼ĖłŹ¾²Ł×÷Ó¦ŌŚĻĀĶ¼ÖŠ__________(ĢīŃ”Ļī×ÖÄø)Ö®¼ä”£
A£®¢ŚÓė¢Ū””””B£®¢ŁÓė¢Ś””””C£®¢ÜÓė¢Ż
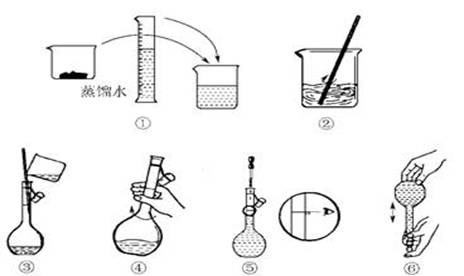
(3) ÅäÖĘ¹ż³ĢÖŠŠčŅŖÓƵ½µÄŅĒĘ÷ÓŠ:ĶŠÅĢĢģĘ½”¢Ņ©³×”¢ÉÕ±”¢ ”¢ ”¢
(4)ČōøĆĶ¬Ń§ŌŚÅäÖĘČÜŅŗŹ±£¬½ųŠŠĮĖČēĻĀ²Ł×÷£¬ĘäÖŠŹ¹ĖłÅäČÜŅŗÅضČĘ«µĶµÄ²Ł×÷
ÓŠ_______________(ĢīŃ”Ļī×ÖÄø)”£
A£®³ĘĮæKMnO4¾§ĢåŹ±£¬ÖøÕėĘ«ĻņÓŅ±ß
B£®½«KMnO4¾§Ģå·ÅŌŚÉÕ±ÖŠČܽā£¬ĄäČ“ŗó£¬×ŖŅĘÖĮŗ¬ÓŠÉŁĮæÕōĮóĖ®µÄČŻĮæĘæÖŠ
C£®¶ØČŻŹ±£¬ŃöŹÓæĢ¶ČĻß
D£®Õńµ“Ņ”ŌČŗ󣬷¢ĻÖČÜŅŗŅŗĆęµĶÓŚæĢ¶ČĻߣ¬ŌŁµĪ¼ÓÉŁĮæÕōĮóĖ®ÖĮæĢ¶ČĻß“¦
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com