
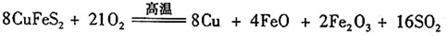


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������þ | B��̼�� | C������ | D��ͭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
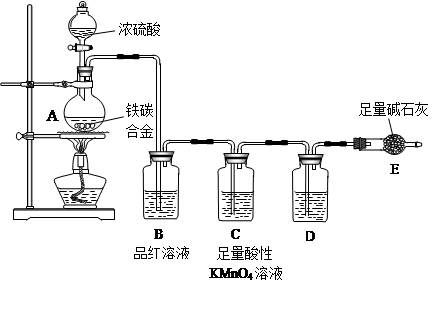
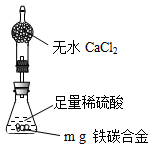
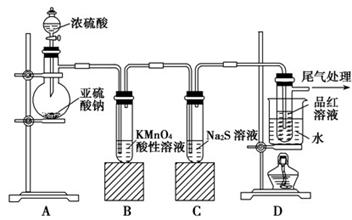
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
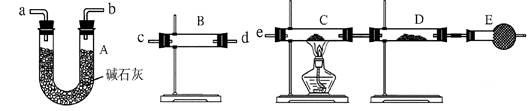
| װ�� | ��ʢҩƷ | ʵ������ | ���ۻ���� |
| B | | | |
| C | CuO���� | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
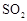 ת��Ϊ
ת��Ϊ ��ת���ʲⶨʵ�飺
��ת���ʲⶨʵ�飺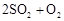

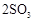 ��
��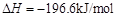 ����֪��
����֪��| | �۵㣨 �� �� | �е㣨 �� �� |
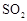 | -72.4 | -10 |
 | 16.8 | 44.3 |
 ��Ϊʹ
��Ϊʹ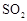 �нϸߵ�ת���ʣ�ʵ��ʱ����Ũ��������ȴ������Ⱥ�˳���� ��
�нϸߵ�ת���ʣ�ʵ��ʱ����Ũ��������ȴ������Ⱥ�˳���� ��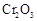 �������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȡ����Է��¶ȹ��ߣ���������ԭ���� �����ô�������
�������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȡ����Է��¶ȹ��ߣ���������ԭ���� �����ô�������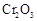 ���ķ�Ӧ��ʱ��
���ķ�Ӧ��ʱ��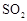 ��ת���ʻ� ������ߡ��������͡����䡱����
��ת���ʻ� ������ߡ��������͡����䡱����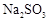 �������Ũ�����������ģ���Ӧ����ʱ����ͨ��
�������Ũ�����������ģ���Ӧ����ʱ����ͨ�� һ��ʱ�䣬�Ƶâ�װ�õ���������11.3g����ʵ����
һ��ʱ�䣬�Ƶâ�װ�õ���������11.3g����ʵ���� ��ת����Ϊ %������С�����һλ����
��ת����Ϊ %������С�����һλ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��ab�η�Ӧ�ǣ�SO2��2H2S===3S����2H2O |
| B���������DZ�������������� |
| C��ԭH2S��Һ�����ʵ���Ũ��Ϊ0.05 mol/L |
| D��b���Ӧ����Һ��������ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
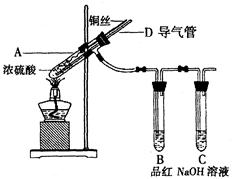
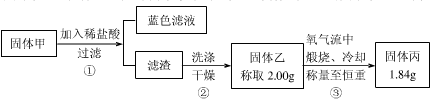
| A��������У�CuS��Cu2S����ͬʱ���� |
| B��������У�CuO��Cu2O������һ�� |
| C�����������û��Cu2O����һ����Cu2S |
| D������������Cu2S |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ�Т� | B���ڢۢܢ� | C���٢ڢۢ� | D���٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Ȼ��� | B�������� | C�������� | D��̼���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com