
| ��� | CaCO3/mol | CaO/mol | CO2/mol |
| A | 0.02 | 0.02 | 0.05 |
| B | 0.02 | 0.02 | 0.03 |
| C | 0.02 | 0.02 | 0.01 |
| D | 0.02 | 0 | 0.05 |
| E | 0.02 | 0.02 | 0 |
���� ��1����֪800��ʱ���÷�Ӧƽ�ⳣ��K=0.003������$\frac{C��CaO����C��C{O}_{2}��}{C��CaC{O}_{3}��}$��K��ƽ�������ƶ���CaCO3��s�����������ӣ�$\frac{C��CaO����C��C{O}_{2}��}{C��CaC{O}_{3}��}$=K����ϵ���ڻ�ѧƽ��״̬��$\frac{C��CaO����C��C{O}_{2}��}{C��CaC{O}_{3}��}$��K��ƽ�������ƶ���CaCO3��s����������С��
��2����Һ�д���H2O?H++OH-��HCO3-?H++CO32-��HCO3-+H2O?OH-+HCO3-����Һ��������Դ��ˮ�ĵ��룬��̼�������ˮ�⣬ˮ�����������Ũ��Ϊc��H+��-c��CO32-����̼�����ˮ��õ�������Ũ�ȵ���̼���Ũ�ȣ��ݴ˽��н��
��3��ˮ���к��е�CaSO4��������̼������Һ������ת��Ϊ CaCO3��
��� �⣺��1����֪800��ʱ���÷�Ӧƽ�ⳣ��K=0.003��A��E��ͬ��Ͷ�Ϸ�ʽ������һ��10L���ܱ������У�
A��$\frac{C��CaO����C��C{O}_{2}��}{C��CaC{O}_{3}��}$=$\frac{0.005��0.002}{0.002}$=0.005��0.003��ƽ�������ƶ���CaCO3��s�����������ӣ�
B��$\frac{C��CaO����C��C{O}_{2}��}{C��CaC{O}_{3}��}$=$\frac{0.003��0.002}{0.002}$=0.003=K����ϵ���ڻ�ѧƽ��״̬��
C��$\frac{C��CaO����C��C{O}_{2}��}{C��CaC{O}_{3}��}$=$\frac{0.001��0.002}{0.002}$=0.001��0.003��ƽ�������ƶ���CaCO3��s����������С��
D��$\frac{C��CaO����C��C{O}_{2}��}{C��CaC{O}_{3}��}$=$\frac{0.005��0}{0.002}$=0��0.003��ƽ�������ƶ���CaCO3��s����������С��
E��$\frac{C��CaO����C��C{O}_{2}��}{C��CaC{O}_{3}��}$=$\frac{0��0.002}{0.002}$=0��0.003��ƽ�������ƶ���CaCO3��s����������С��
�ʴ�Ϊ��A��C��D��E��B��
��2����Һ�д���H2O?H++OH-��HCO3-?H++CO32-��HCO3-+H2O?OH-+HCO3-����Һ��������Դ��ˮ�ĵ�����̼�������ˮ�⣬ˮ�����������Ũ��Ϊc��H+��-c��CO32-����̼�����ˮ��õ�������Ũ�ȵ���̼���Ũ�ȣ�����Һ��c��OH-��=c��H+��+c��H2CO3��-c��CO32-����
�ʴ�Ϊ��c��H+��+c��H2CO3��-c��CO32-����
��3��ˮ���к��е�CaSO4��������̼������Һ������ת��Ϊ CaCO3��ת�������ӷ���ʽ��CaSO4��s��+CO32- ��aq��?CaCO3��s ��+SO42- ��aq����
�ʴ�Ϊ��CaSO4��s��+CO32- ��aq��?CaCO3��s ��+SO42- ��aq����
���� ���⿼�黯ѧƽ���Ӱ�����ء�����Ũ�ȵıȽϡ�����ת���ȣ���2��Ϊ�״��㡢�ѵ㣬ѧ��������ˮ����Ŀ�Ѷ��еȣ�


 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
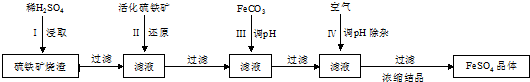
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
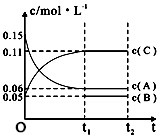
| A�� | ��a=3����b=1��c=2 | |
| B�� | t1minʱ���÷�Ӧ�ﵽ�������µķ�Ӧ�� | |
| C�� | ��O��t1min�ڣ���C��ʾ�Ļ�ѧ��Ӧ����Ϊ0.06mol•L-1 | |
| D�� | B����ʼŨ�ȵ���0.08mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ʹ������к͵ζ����ⶨ���۰״���������g/100mL����
ʹ������к͵ζ����ⶨ���۰״���������g/100mL����| �ζ����� ʵ�����ݣ�mL�� | 1 | 2 | 3 | 4 |
| V����Ʒ�� | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH�������ģ� | 15.95 | 15.00 | 15.05 | 14.95 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
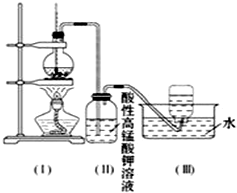 ��ͼ��ʾ����ʵ�����ͨ������Ũ�������Ҵ���ϼ�������ϩ������һ��ʱ�����Һ���к�ɫ������֣���һ��ʱ��������ữ�ĸ��������Һ��ɫ����������֪�������������к���CH2=CH2��SO2��CO2��H2O��
��ͼ��ʾ����ʵ�����ͨ������Ũ�������Ҵ���ϼ�������ϩ������һ��ʱ�����Һ���к�ɫ������֣���һ��ʱ��������ữ�ĸ��������Һ��ɫ����������֪�������������к���CH2=CH2��SO2��CO2��H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Fe2++2NH3•H2O�TFe��OH��2��+2NH4+ | |
| B�� | Fe2++NH3•H2O+HCO3-�TFeCO3��+NH4++H20 | |
| C�� | Fe2++2HCO3-�TFe��OH��2��+2CO2�� | |
| D�� | 2Fe2++HCO3-+3NH3•H2O�TFe2��OH��2CO3��+3NH4++H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ش��������⣮
��ش��������⣮| ��ѧʽ | ����ƽ�ⳣ����25�棩 |
| HCN | K=4.9��10-10 |
| CH3COOH | K=1.8��10-5 |
| H2CO3 | K1=4.3��10-7��K2=5.6��10-11 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com