
�Ҵ�����Ҫ���л�����ԭ�ϣ�������ϩ����ֱ��ˮ�Ϸ���������ˮ�Ϸ��������ش��������⣺
(1)���ˮ�Ϸ���ָ�Ƚ���ϩ��Ũ���ᷴӦ��������������(C2H5OSO3H)����ˮ�������Ҵ���д����Ӧ��Ӧ�Ļ�ѧ����ʽ��________________________________________��
(2)��֪��
�״�����ˮ��Ӧ
2CH3OH(g)===CH3OCH3(g)��H2O(g)
��H1����23.9 kJ��mol��1
�״���ϩ���ķ�Ӧ
2CH3OH(g)===C2H4(g)��2H2O(g)
��H2����29.1 kJ��mol��1
�Ҵ����칹����Ӧ��C2H5OH(g)===CH3OCH3(g)
��H3����50.7 kJ��mol��1
����ϩ����ֱ��ˮ�Ϸ�ӦC2H4(g)��H2O(g)===C2H5OH(g)�Ħ�H________kJ��mol��1������ˮ�Ϸ���ȣ�����ֱ��ˮ�Ϸ����ŵ���____________________________________��
(3)��ͼ��ʾΪ����ֱ��ˮ�Ϸ�����ϩ��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ[����n(H2O)��n(C2H4)��1��1]��
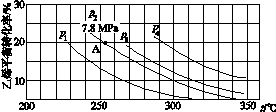
����ʽ������ϩˮ�����Ҵ���Ӧ��ͼ��A���ƽ�ⳣ��Kp��____________________(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������)��
��ͼ��ѹǿ(p1��p2��p3��p4)�Ĵ�С˳��Ϊ____��������___________________��
������ֱ��ˮ�Ϸ������õĹ�������Ϊ������/������Ϊ��������Ӧ�¶�290 �桢ѹǿ6.9 MPa��n(H2O)��n(C2H4)��0.6��1����ϩ��ת����Ϊ5%����Ҫ��һ�������ϩת���ʣ����˿����ʵ��ı䷴Ӧ�¶Ⱥ�ѹǿ�⣬���ɲ�ȡ�Ĵ�ʩ��________________________________________________________________________��
________________________________________________________________________��
(1)C2H4��H2SO4�D��C2H5OSO3H��C2H5OSO3H��H2O�D��C2H5OH��H2SO4��(2)��45.5����ȾС����ʴ��С�ȡ�(3)�� ��
��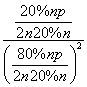 ��
��
 ��0.07(MPa)��1����p1<p2<p3<p4����Ӧ���������٣���ͬ�¶��£�ѹǿ������ϩת�������
��0.07(MPa)��1����p1<p2<p3<p4����Ӧ���������٣���ͬ�¶��£�ѹǿ������ϩת�������
�۽������Ҵ�Һ����ȥ������n(H2O)��n(C2H4)��
[����] (1)����������Ϣ��д������ϩ��Ũ������ˮ�Ϸ����Ҵ��ķ�ӦΪC2H4��H2SO4�D��C2H5OSO3H ��C2H5OSO3H��H2O�D��C2H5OH��H2SO4��(2)���ݸ�˹���ɢ٣��ڣ��۵ã�C2H4(g)��H2O(g)===C2H5OH(g)����H����45.5 kJ��mol��1�����ˮ�Ϸ����õ�Ũ�����ǿ��ʴ�����ʣ��������ֱ��ˮ�Ϸ�������ȾС����ʴ��С���ŵ㡣(3)������ʼʱC2H4��H2O(g)�����ʵ�����Ϊn������C2H4��ת����Ϊ20%����ƽ��ʱC2H4��H2O(g)��C2H5OH�����ʵ����ֱ�Ϊ80%n��80%n��20%n����Kp�� ��
��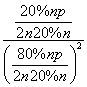 ��
�� ��0.07(MPa)��1��������ѹǿ��ƽ�⽫�����ƶ��������C2H4��ת���ʣ���ѹǿp1��p2��p3��p4����Ϊ��ʹƽ�������ƶ��������Խ��Ҵ�Һ����ʱ���룬������n (H2O)��n (C2H4) ֮�ȵȴ�ʩ��
��0.07(MPa)��1��������ѹǿ��ƽ�⽫�����ƶ��������C2H4��ת���ʣ���ѹǿp1��p2��p3��p4����Ϊ��ʹƽ�������ƶ��������Խ��Ҵ�Һ����ʱ���룬������n (H2O)��n (C2H4) ֮�ȵȴ�ʩ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪X��Y��ZΪ���ʣ�����Ϊ���������YΪ���壬G��Һ�ʻ�ɫ��E�Dz�����ˮ�������������������ᷴӦ������֮�������ͼK133��ʾ��ת����ϵ(���ֲ�������ȥ)��
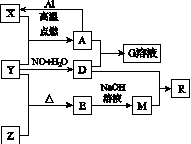
ͼK133
��ش��������⣺
(1)д������E��һ����;��________________________________________________________________________��
(2)д��A��D��ϡ��Һ��Ӧ����G�����ӷ���ʽ��
__________________________________________��
(3)��4 mol D��ϡ��Һ�У�����X��ĩ��һ����������D��ȫ��Ӧ�����ɵ�����ֻ��һ�֣���Ӧ��X�����ʵ�����ΧΪ________mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ú̿ȼ�չ����л��ͷų�������SO2�������ƻ���̬����������һ����������������Ԫ����CaSO4����ʽ�̶����Ӷ�����SO2���ŷš�����ú̿ȼ�չ����в�����CO�ֻ���CaSO4������ѧ��Ӧ����������Ч�ʡ���ط�Ӧ���Ȼ�ѧ����ʽ���£�
CaSO4(s)��CO(g) CaO(s) �� SO2(g) �� CO2(g)����H1��218.4 kJ��mol��1(��Ӧ��)
CaO(s) �� SO2(g) �� CO2(g)����H1��218.4 kJ��mol��1(��Ӧ��)
CaSO4(s)��4CO(g) CaS(s) �� 4CO2(g)����H2����175.6 kJ��mol��1(��Ӧ��)
CaS(s) �� 4CO2(g)����H2����175.6 kJ��mol��1(��Ӧ��)
��ش��������⣺
(1)��Ӧ���ܹ��Է����еķ�Ӧ������________��
(2)�����������ķ�Ӧ����ʾƽ�ⳣ��Kpʱ���������(B)��ƽ��ѹǿp(B)������������ʵ�����Ũ��c(B)����Ӧ���Kp��________(�ñ���ʽ��ʾ)��
(3)����ij�¶��£���Ӧ�������(v1 )���ڷ�Ӧ�������(v2 )�������з�Ӧ���������仯ʾ��ͼ��ȷ����________��
(4)ͨ����ⷴӦ��ϵ������Ũ�ȵı仯���жϷ�Ӧ��͢��Ƿ�ͬʱ������������____________________________________________________________________��
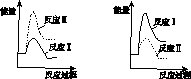
�������������� ��A������������B
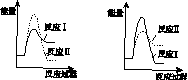
�������������� ��C������������D
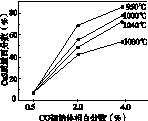
(5)ͼ(a)Ϊʵ���ò�ͬ�¶��·�Ӧ��ϵ��CO��ʼ����ٷ�����ƽ��ʱ���������CaS�����ٷ����Ĺ�ϵ���ߡ��÷�Ӧ��ϵ��SO2�������Ĵ�ʩ��________��
A����÷�Ӧ��ϵ��Ͷ��ʯ��ʯ
B���ں��ʵ��¶�������ƽϵ͵ķ�Ӧ�¶�
C�����CO�ij�ʼ����ٷ���
D����߷�Ӧ��ϵ���¶�
(6)���º��������£����跴Ӧ��͢�ͬʱ��������v1>v2������ͼ(b)������Ӧ��ϵ��c(SO2)��ʱ��t�仯��������ͼ��
 ��
��
����������(a)������������������������(b)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳɰ��������ѧ�����ϵ�һ���ش�ͻ�ƣ��䷴Ӧԭ��Ϊ
N2(g)��3H2(g)  2NH3(g)����H����92.4 kJ��mol��1��
2NH3(g)����H����92.4 kJ��mol��1��
һ�ֹ�ҵ�ϳɰ��ļ�ʽ����ͼ���£�
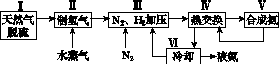
(1)��Ȼ���е�H2S���ʳ��ð�ˮ���գ�����ΪNH4HS��һ����������NH4HS��Һ��ͨ��������õ�������ʹ����Һ������д��������Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
(2)���������������ԭ�����£�
��CH4(g)��H2O(g)  CO(g)��3H2(g)
CO(g)��3H2(g)
��H����206.4 kJ��mol��1
��CO(g)��H2O(g)  CO2(g)��H2(g)
CO2(g)��H2(g)
��H����41.2 kJ��mol��1
���ڷ�Ӧ�٣�һ���������ƽ����ϵ��H2�İٷֺ��������ܼӿ췴Ӧ���ʵĴ�ʩ��____________��
a�������¶ȡ�b������ˮ����Ũ�ȡ�c�����������d������ѹǿ
���÷�Ӧ�ڣ���CO��һ��ת���������H2�IJ�������1 mol CO��H2�Ļ������(CO���������Ϊ20%)��H2O��Ӧ���õ�1.18 mol CO��CO2��H2�Ļ�����壬��CO��ת����Ϊ____________��
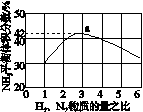
(3)ͼ(a)��ʾ500 �桢60.0 MPa�����£�ԭ����Ͷ�ϱ���ƽ��ʱNH3��������Ĺ�ϵ������ͼ��a�����ݼ���N2��ƽ�����������____________��
(4)�����¶ȶԺϳɰ���Ӧ��Ӱ�죬��ͼ(b)����ϵ�У�����һ�������µ��ܱ������ڣ���ͨ��ԭ������ʼ�����¶Ȳ������ߣ�NH3���ʵ����仯������ʾ��ͼ��
 ��
��
(a)������������������(b)
(5)��������ͼ�У�ʹ�ϳɰ��ų��������õ�������õ���Ҫ������(�����)________����������������ߺϳɰ�ԭ����ת���ʵķ�����________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������AX3�͵���X2��һ�������·�Ӧ�����ɻ�����AX5���ش��������⣺
(1)��֪AX3���۵�ͷе�ֱ�Ϊ��93.6 ���76 �棬AX5���۵�Ϊ167 �档����ʱAX3������X2��Ӧ����1 mol AX5���ų�����123.8 kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ____________________________________________��
(2)��ӦAX3(g)��X2(g)  AX5(g)���ݻ�Ϊ10 L���ܱ������н��С���ʼʱAX3��X2��Ϊ0.2 mol����Ӧ�ڲ�ͬ�����½��У���Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ��
AX5(g)���ݻ�Ϊ10 L���ܱ������н��С���ʼʱAX3��X2��Ϊ0.2 mol����Ӧ�ڲ�ͬ�����½��У���Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ��
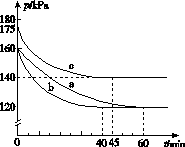
����ʽ����ʵ��a�ӷ�Ӧ��ʼ���ﵽƽ��ʱ�ķ�Ӧ����v(AX5)��______________________��
��ͼ��3��ʵ��ӷ�Ӧ��ʼ���ﵽƽ��ʱ�ķ�Ӧ����v(AX5)�ɴ�С�Ĵ���Ϊ____________(��ʵ�����)����ʵ��a��ȣ���������ı��ʵ���������ж������ǣ�b________________________________________________��c____________________________________________��
����p0��ʾ��ʼʱ��ѹǿ��p��ʾƽ��ʱ��ѹǿ������ʾAX3��ƽ��ת���ʣ������ı���ʽΪ______________��ʵ��a��c��ƽ��ת���ʣ���aΪ________����cΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2.0 L�����ܱ������г���1.0 mol PCl5�����¶�ΪTʱ������ӦPCl5(g) 
 PCl3(g)��Cl2(g)����H����124 kJ��mol��1����Ӧ�����вⶨ�IJ������ݼ��±���
PCl3(g)��Cl2(g)����H����124 kJ��mol��1����Ӧ�����вⶨ�IJ������ݼ��±���
| ʱ��t/s | 0 | 50 | 150 | 250 | 350 |
| n(PCl3)/mol | 0 | 0.16 | 0.19 | 0.2 | 0.2 |
�ش��������⣺
(1)��Ӧ��ǰ50 s��ƽ������v(PCl5)��____________��
(2)�¶�ΪTʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ����________��
(3)Ҫ���������Ӧ��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��______________��
(4)���¶�ΪTʱ������ʼʱ�������г���0.5 mol PCl5��a mol Cl2ƽ��ʱPCl5��ת������Ϊ20%����a��________��
(5)����ˮ�У����Ȼ�����ȫˮ�⣬��������(H3PO4)���÷�Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
����0.01 mol PCl5Ͷ��1 L��ˮ�У�����μ���AgNO3��Һ���Ȳ����ij�����________[��֪Ksp(Ag3PO4)��1.4��10��16��Ksp(AgCl)��1.8��10��10]��
(6)һ�������£���������������Һ����һ�ֵ������������壬���ɸ����������ĵİ������ʵ���֮��Ϊ20��3����Ӧ�Ļ�ѧ����ʽΪ______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�ֽ�1 mol H2O2�ų�����98 kJ���ں�����I������Һ�У�H2O2�ֽ�Ļ���Ϊ
H2O2��I���D��H2O��IO����������
H2O2��IO���D��H2O��O2��I������
�����йظ÷�Ӧ��˵����ȷ����(����)
A����Ӧ������I��Ũ���й�
B��IO��Ҳ�Ǹ÷�Ӧ�Ĵ���
C����Ӧ��ܵ���98 kJ��mol��1
D��v(H2O2)��v(H2O)��v(O2)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��
����ӵ�ص��ܷ�ӦΪ
LixC��Li1��xCoO2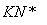 C��LiCoO2
C��LiCoO2
����ص��ܷ�Ӧ2Li��S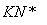 Li2S
Li2S
�й��������ֵ��˵����ȷ����(����)
A������ӵ�طŵ�ʱ�� Li����Ǩ��
B������س��ʱ��﮵缫������ԭ��Ӧ
C�����������ֵ�صı�������ͬ
D����ͼ��ʾ������ӵ�ظ�����س��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com