
���л�������У���ͬλ�õ���ԭ�ӵĺ˴Ź������и�����������ֵ(�ź�)Ҳ��ͬ�����ݷ�ֵ(�ź�)����ȷ���л����������ԭ�ӵ��������Ŀ��
(1)�����л�������У���˴Ź�������ͼ��ֻ��1�����������________��
| A��CH3��CH3 | B��CH3COOH | C��CH3COOCH3 | D��CH3COCH3 |

(1)A D (2) CH2BrCH2Br 2
��������������˴Ź����������м����壬�л����о��м�����ԭ�ӣ��������ȵ�����ԭ�ӵĸ����ȡ������Ҫ�����Ч��ĸ����ͬһ��̼ԭ���ϵ����Ч���磺���顣��ͬһ��̼ԭ���������ϵ���ԭ�ӵ�Ч����2��2-�������飬�������顣�۶Գ������˶ԳƵ���ԭ�ӵ�Ч����������ֻ���������⡣
��1���л���˴Ź�������ͼ��ֻ��1����˵���л���ֻ��һ���⣬��ϵ�Ч��ĸ���ѡA��D��
��2������ʽ��C2H4Br2���л��������ֽṹCH2BrCH2Br��CH3CHBr2�����A�ĺ˴Ź���������ֻ��һ���壬��ṹ��ʽΪCH2BrCH2Br��B�ṹ��ʽΪCH3CHBr2����������ԭ�Ӻ˴Ź�������ͼ����2���塣
���㣺����˴Ź�������ʶ���л�������ԭ��������ж�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�л���A1��A2�ֱ��ŨH2SO4��һ���¶��¹��ȶ�������B��B�������ܶ���ͬ��ͬѹ��H2�ܶȵ�59�����ڴ��������£�1mol B���Ժ�4mol H2�����ӳɷ�Ӧ��B��һԪ�������������֣�ͬ�����ͣ����й�����֮���ת����ϵ���£���[O]��ʾ������Ӧ��
��1����Ӧ������ ��Ӧ����Ӧ������ ��Ӧ
��2��д��A2��X�������ʵĽṹ��ʽ��
A2 X
��3����д��ѧ����ʽ��
��
��
��4��������E�ж���ͬ���칹�壬��д���������������Ҿ���������λ������ͬ���칹��Ľṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪�����л���ѧ��Ӧ��Ӧ������ͬ�������ɲ�ͬ���л���Ʒ�����磺
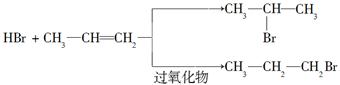
�ڱ���ͬϵ����±�ص��ʻ�ϣ����ڹ��������£����������ϵ���ԭ�ӱ�±��ԭ��ȡ�������ڴ��������£�����ijЩλ���ϵ���ԭ�ӱ�±��ԭ��ȡ����
��ҵ������������Ϣ��������·�ߺϳɽṹ��ʽΪ�����ʣ���������һ�����ϡ�


���������·�ߣ��ش��������⣺
(1)A�Ľṹ��ʽ����Ϊ_____________��____________________��
(2)��Ӧ�۵ķ�Ӧ����Ϊ_________________����Ӧ�ݵ�������_______________
(3)��Ӧ�ܵĻ�ѧ����ʽΪ(�л���д�ṹ��ʽ����ע����Ӧ��������ͬ)��
__________________________________________________��
(4)��ҵ�����У��м����A�뾭��Ӧ�ۢܢݵ�D��������ȡ��Aֱ��ת��ΪD�ķ�������ԭ����____________________________��
(5)�����Ʒ�й����ŵĻ�ѧ��Ӧ����ʽΪ��_____________________________��
(6)�������Ͼ��ж���ͬ���칹�壬����ijЩ��������������������ˮ��Һ��FeCl3��Һ����ɫ���� �����ϵ�һ����������֣��۷�����û�м���д��һ�ַ����������������ʿ��ܵĽṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ�д�������ƽ�⣺Br2+H2O HBr+HBrO�л���Ӧ�����Ƚϸ��ӣ����渱��Ӧ��������֪X���ӳɷ�Ӧ�������ɲ���A��D�����������������ա�
HBr+HBrO�л���Ӧ�����Ƚϸ��ӣ����渱��Ӧ��������֪X���ӳɷ�Ӧ�������ɲ���A��D�����������������ա�
��1��A��B�ķ�Ӧ����Ϊ_____________��д��C�Ľṹ��ʽ_________________��
��2����C��ͬ���칹��M�����ܷ���������Ӧ��Ҳ�ܷ���������Ӧ��M��_____��ͬ���칹�壬д�����з��ӽṹ����֧���ķ��ӵĽṹ��ʽ___________________,
��3��д�������л���Ľṹ��ʽ�� F_________________��I______________��
��4�����G��H��ѧ����ʽ��
__________________________________________��
��5�����X��D��ѧ����ʽ__________________________________________��
��6��X����ˮ�ķ�Ӧ�л�����һ���л��������ṹ��ʽΪ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������������֮���ת����ϵ���ش����⣺
��1������ȡ����Ӧ���У�����ţ���ͬ��___________�����ڼӳɷ�Ӧ���� ____ _____��
��2��д����Ӧ�ۡ��ܵĻ�ѧ����ʽ(�л����ýṹ��ʽ��ʾ��ע����Ӧ��������ͬ)
a����Ӧ��___________________________________________________________��
b����Ӧ��__________________________________________________________��
��3��д�� ������������ͭ����Һ(NaOH���Ի���)��Ӧ�Ļ�ѧ����ʽ
������������ͭ����Һ(NaOH���Ի���)��Ӧ�Ļ�ѧ����ʽ
___________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������Diels��Alder��Ӧ��һ��ʵ����
��������������仯���ش��������⣺
��ij����AΪ��ʼԭ�Ϻϳɻ�����G��·�����£�ͼ��Mr��ʾ��Է�����������
��1��ָ����Ӧ���ͣ�B��C________��F��G_________��
��2��д���������ʵĽṹ��ʽ��A______________��F_____________��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��B��C________��D��E___________��
��4��C��D��ת������һ��ʵ�֣������C��D�ĺϳ�·�ߣ�_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A�Ǻϳ���Ȼ�ĵ��壬����ʽΪC5H8��A��һϵ�з�Ӧ����(���ַ�Ӧ����ʡ��)��
�ش��������⣺
��1��A�������� ��B�Ľṹ��ʽΪ ��
��2���ڵķ�Ӧ����ʽΪ ��
��3��A��ͬ���칹��������Ȳ�����칹���� �֡�
��4��A��B��Ӧ����������һ����Ԫ��������Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������M��ij�ֽ�����ʹҩ����AΪԭ�ϵĹ�ҵ�ϳ�·������ͼ��ʾ��
|
 ��֪��
��֪��  +
+

 +CH3COOH
+CH3COOH
 ����һ����ɣ�����Ϳ��ܵ�ԭ��
����һ����ɣ�����Ϳ��ܵ�ԭ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����л������ﻯѧ���ʵ�ԭ�ӻ�ԭ���Ž��������ţ���±ԭ�ӣ���X������������NH2����̼̼��������C��C�����ȡ�
��1����ȩ����������Ӧ��˵����ȩ�����к��� ��д���ƣ������š�
��2��ˮ����Ľṹ��ʽΪ�� ������ �ֹ����š�
������ �ֹ����š�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com