
Na2SO3�����ڿ����о������ױ��ʡ�
I��д��Na2SO3�����ڿ����б��������ʵĻ�ѧ����ʽ_______________��
��ʵ������������ Na2SO3 ������Ʒ�Ƿ��Ѿ����ʣ���ķ���Ϊ_____________��
��ѡ������װ�ú�ҩƷͨ���ⶨ����SO2���������ɼ���Na2SO3������Ʒ�Ĵ��ȡ�
��Ӧԭ����H2SO4(Ũ)+Na2SO3=Na2SO4+SO2��+H2O
��֪��ʯ��������SO2
��ѡ�õ�ҩƷ����Ũ���� ��Ũ���� �ۼ�ʯ�� �ܿ���

��1������ѡ����������˳������������������±�������Ӧ����������������Լ���������Aװ����ҩƷ��������Ҫ��д���ɲ�������

��2����ȡagNa2SO3������Ʒ������ʵ����ɺ������SO2������Ϊbg�����Na2SO3������Ʒ��������Ϊ_____________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������������ѧ�߶�ʵ��������л�ѧ���������棩 ���ͣ�ѡ����
����������������ѧ���״κϳɵ�117��Ԫ�أ���������ʱ�����ܿ���ѡΪ����ʮ���ѧ���֡������ 117 ��Ԫ�ط�����ʱ��ΪUp�����й���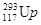 ��
�� ˵������ȷ����
˵������ȷ����
A. 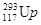 ��
�� ������Ԫ��
������Ԫ��
B. 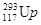 ��
�� ��Ϊͬλ��
��Ϊͬλ��
C. 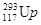 ��
�� ��������ͬ����������ͬ
��������ͬ����������ͬ
D. 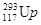 ��
�� ��������ͬ����������ͬ
��������ͬ����������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ�߶������л�ѧ�Ծ��������棩 ���ͣ�ѡ����
ȷ��ȡ20.00 mLij����HCl��Һ����ƿ�У���0.100 0 mol��L��1NaOH��Һ�ζ��� ����˵����ȷ����( )
A.�ζ���������ˮϴ�Ӻ�װ��NaOH��Һ���еζ�
B.����NaOH��Һ���룬��ƿ����ҺpH��С���
C.�÷�̪��ָʾ��������ƿ����Һ�ɺ�ɫ����ɫʱֹͣ�ζ�
D.�ζ����յ�ʱ�����ֵζ��ܼ��첿�������Σ���ⶨ���ƫС
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�������¿�����ѧ�Ծ��������棩 ���ͣ������
[��ѧ����ѡ��5���л���ѧ����]
��ˮ����Ľṹ��ʽΪ ��
��
��1�����й���ˮ�����������ȷ����_______________��
A�� ��
�� ��Ϊͬϵ��
��Ϊͬϵ��
B��ˮ�������������ԭ��һ������ͬһƽ����
C��ˮ����ȿ��Կ����Ƿ������ʣ�Ҳ���Կ���������������
��2����ˮ������______________��Һ���ã��������� ����д����
����д���� ת��Ϊ
ת��Ϊ �Ļ�ѧ����ʽ_______________��
�Ļ�ѧ����ʽ_______________��
��������������֪ij��״ϩ������Է�������Ϊ124��������KMnO4��Һ�������õ����ֲ��a��CH3COOH�� b��
R1-CH=CH-R2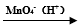 R1-COOH + R2-COOH
R1-COOH + R2-COOH
��3��a������Ϊ________________��
��4��д���������ܵĽṹ��ʽ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�������¿�����ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪����������Һ�м����������ʱ������Ӧ��4Fe2+ +4Na2O2 +6H2O=4Fe(OH)3��+O2��+8Na+��������˵����ȷ����
A���÷�Ӧ��Na2O2�������������ǻ�ԭ����O2�ǻ�ԭ����
B����Fe2+ʧȥ4mol���ӣ��������������ԼΪ22.4L
C��4 mol Na2O2�ڷ�Ӧ�й��õ�6NA������
D����Ӧ�����п��Կ�����ɫ������ת��Ϊ����ɫ��ת��Ϊ���ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��������������ѧԺ���и�һ�����л�ѧ���������棩 ���ͣ�ѡ����
����������NF3����һ����ɫ����ζ�����壬�������ӹ�ҵ�����Ĺؼ�ԭ��֮һ�����ɰ����ͷ�����Ӧ�õ���4NH3+3F2=NF3+3NH4F���ڳ�ʪ�Ŀ����У�NF3����ˮ��������������ԭ��Ӧ���䷴Ӧ�IJ�����HF��NO��HNO3(NO���������ɺ���ɫ��NO2)������˵����ȷ����
A����NF3��H2O�ķ�Ӧ�У�H2O����ԭ��
B����ȡNF3�ķ�Ӧ�У��������뻹ԭ�������ʵ���֮��Ϊ3��1
C��������ȡNF3�ķ�Ӧ����0.5mol NH3�μӷ�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ3mol
D��NF3�ڳ�ʪ�Ŀ�����һ��й©�����ױ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��������������ѧԺ���и�һ�����л�ѧ���������棩 ���ͣ�ѡ����
������Һ��c(Cl-)��50mL1mol/LAlCl3��Һ��c(Cl-)��ȵ���
A��150mL 1mol/L��NaCl��Һ B��75mL 2mol/L��NH4Cl��Һ
C��150mL 2mol/L��KCl��Һ D��75mL 1mol/L��FeCl3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ĸ�һ���¿�����ѧ���������棩 ���ͣ������
ѡ��һ���Լ���ȥ���и������е����ʣ�������Ϊ���ʣ�������ѡ�Լ��Ļ�ѧʽ���ں����ϣ���д�����ӷ���ʽ��
��1��BaCl2(HCl)�Լ� �����ӷ���ʽΪ ��
��2��CO(CO2)�Լ� �����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ĸ߶����¿�����ѧ���������棩 ���ͣ�ѡ����
������ֽʹ��ʱ����������ˮ��ʪ���ǣ� ��
A. pH��ֽ B. ��ɫʯ����ֽ C. ���۵⻯����ֽ D. ��ɫʯ����ֽ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com