
| A�� | �����£�pH=9��NaHA��Һ��c��Na+����c��HA-����c��H2A�� | |
| B�� | Na2CO3��Һ��c��H+��-c��OH-��=c��HCO3-��+2c��CO32-��-c��Na+�� | |
| C�� | ��NaOH��Һ�е���CH3COOH��Һ����Һ�Լ��ԣ�c��CH3COO-����c��OH-����c��H+�� | |
| D�� | Ũ�Ⱦ�Ϊ0.1mol•L-1��HF��Һ��KF��Һ�������ϣ�c��F-��+c��HF��=0.2mol•L-1 |
���� A��NaHA��Һ��pH=9��˵����Һ�ʼ��ԣ�HA-ˮ�����HA-����̶ȣ�
B�����ݵ���غ�������
C������������ƴ�����ʣ����c��OH-����c��CH3COO-����c��H+����
D�����������غ�������
��� �⣺A��NaHA��Һ��pH=9��˵����Һ�ʼ��ԣ�HA-ˮ�����HA-����̶ȣ���c��Na+����c��HA-����c��H2A����c��A2-������A��ȷ��
B��Na2CO3��Һ���ɵ���غ��֪��c��Na+��+c��H+��=c��HCO3-��+2c��CO32-��+c��OH-��������c��H+��-c��OH-��=c��HCO3-��+2c��CO32-��-c��Na+������B��ȷ��
C������������ƴ�����ʣ����c��OH-����c��CH3COO-����c��H+������C����
D��Ũ�Ⱦ�Ϊ0.1mol•L-1��HF��Һ��KF��Һ�������ϣ�c��F-��+c��HF��=$\frac{0.1V+0.1V}{2V}$=0.1mol•L-1����D����
��ѡAB��
���� ���⿼������Ũ�ȵĴ�С�ıȽϣ�ѧ��Ҫѧ�����غ�������غ��ڽ�����Ũ�ȴ�С�����е��ۺ�Ӧ�ã��Ƚ����ף�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ϊ��ԭ�ӱ���ԭ��ʧȥ������Ŀ�࣬���������ƵĻ�ԭ��ǿ | |
| B�� | ij���Ľṹʾ���ͼΪ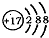 �����Ԫ�������ڱ���λ�ڵ������ڡ�VIA�� �����Ԫ�������ڱ���λ�ڵ������ڡ�VIA�� | |
| C�� | Be��OH��2�ļ��Ա�Mg��OH��2�ļ���ǿ | |
| D�� | ԭ�Ӱ뾶��Na��Si��O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ˮ�еμ�ŨH2SO4��KW���� | |
| B�� | 25��ʱ���� c��H+��=$\sqrt{{K}_{W}}$����Һ�У���������Na+��S2-��NO3-��CO32-������ | |
| C�� | ���£�0.1mol•L-1��NaHSO3��Һ��pH=6����c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-�� | |
| D�� | NaCl��Һ��CH3COONH4��Һ�������ԣ�����Һ��ˮ�ĵ���̶���ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1 mol•L-1NaClO��Һ�к��е�ClO-��ĿΪNA | |
| B�� | ���³�ѹ�£�11.2 L Cl2����ԭ����ΪNA | |
| C�� | һ�������£���1 mol N2��3 mol H2��ϣ���ַ�Ӧ��ת�Ƶĵ�����Ϊ6NA | |
| D�� | 6.4 g��S2��S4��S8��ɵĻ���ﺬ��ԭ����Ϊ0.2 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 һ�������£��̶��ݻ����ܱ������У�CO��H2��Ӧ���ɼ״���CO��g��+2H2��g��?CH3OH��g������ͼ�Ǹ÷�Ӧ�ڲ�ͬ�¶���CO��ת������ʱ��仯�����ߣ������ж���ȷ���ǣ�������
һ�������£��̶��ݻ����ܱ������У�CO��H2��Ӧ���ɼ״���CO��g��+2H2��g��?CH3OH��g������ͼ�Ǹ÷�Ӧ�ڲ�ͬ�¶���CO��ת������ʱ��仯�����ߣ������ж���ȷ���ǣ�������| A�� | T1��T2���÷�Ӧ�ġ�H��0 | |
| B�� | ��������������CO��ת���� | |
| C�� | T1ʱ��ƽ�ⳣ��K1��T2ʱ��ƽ�ⳣ��K2 | |
| D�� | CO��g��+2H2��g��?CH3OH��1���ġ�Hֵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ˮ���ӵı���ģ�ͣ� | B�� | F-�Ľṹʾ��ͼ�� | ||
| C�� | H2O2�ĵ���ʽ��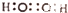 | D�� | �����ǵĽṹ��ʽ��C6H12O6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ��Ŀ�� | ʵ�����ƻ���� |
| A | �Ƚ�Cl��SԪ�صķǽ�����ǿ�� | ��ͬ�����£��ⶨ��ͬŨ�ȵ�NaCl��Һ��Na2S��Һ��pH |
| B | �Ƚ�Ksp��AgCl����Ksp��AgI���Ĵ�С | ��AgCl��Һ�е���������KI��Һ���� |
| C | ����ȥ���л��еı��� | ����Һ�м���Ũ��ˮ����ַ�Ӧ���� |
| D | ֤��������H2O2��Fe3+ǿ | �������ữ��H2O2��Һ����Fe��NO3��2��Һ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
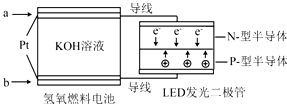
| A�� | b��ͨ��H2 | |
| B�� | ��װ�ý���ѧ������ת��Ϊ���� | |
| C�� | ͨ��H2�ĵ缫������Ӧ��2H2-4e-=4H+ | |
| D�� | a��Ϊ��ظ�����������������Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com